A case report of high-grade muscle-invasive bladder cancer undergoing radical cystectomy after neoadjuvant therapy with RC48
-
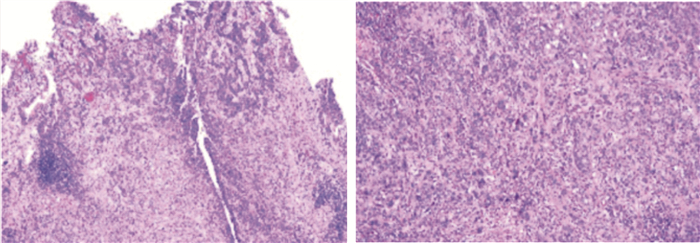
摘要: 报道1例高级别浸润性膀胱癌在维迪西妥单抗新辅助治疗后行根治性膀胱切除术(RC)的治疗效果,探讨新辅助治疗对此类患者的临床意义。1例70岁男性膀胱癌患者,因多支冠状动脉(冠脉)狭窄,需行冠脉旁路移植术(CABG),在CABG围手术期(3个月内)暂时无法耐受RC手术。患者在局麻下行膀胱镜检查术,活检病理提示:高级别尿路上皮癌,HER2(2+),PD-L1(CPS=2)。综合考虑患者肿瘤的病理类型、免疫组化结果以及患者经济情况,对患者行维迪西妥单抗(RC48)新辅助治疗,CABG术后3个月行RC手术。结果显示,患者在CABG术后2周开始行维迪西妥单抗新辅助治疗,每次120 mg,每2周1次,共治疗4次,期间复查2次CTU显示肿瘤负荷明显缩小,达到部分缓解,疾病得到了有效控制。在CABG术后3个月,对患者行RC手术。术后病理检查提示膀胱高级别浸润性尿路上皮癌,T2bN0M0,切缘阴性。维迪西妥单抗为代表的抗体药物偶联物的新辅助治疗具有光明的前景,值得进一步探究。Abstract: We reported the efficacy of radical cystectomy (RC) after neoadjuvant therapy with RC48 for a case of high-grade muscle-invasive bladder cancer so as to explore the clinical significance of neoadjuvant therapy for this kind of patients. A 70-year-old male patient with bladder cancer underwent coronary artery bypass grafting (CABG) due to multiple coronary artery stenosis. During the perioperative period of CABG (within 3 months), RC was temporarily unable to be tolerated. Cystoscopy was performed under local anesthesia. Considering the pathological type of the tumor, the results of immunohistochemistry and the economic situation of the patient, the patient was treated with RC48 neoadjuvant therapy, and RC was performed three months after CABG. Then two weeks after CABG, the patient was treated with RC48 neoadjuvant therapy, 120mg each time, once every 2 weeks, for four times. During the period of RC48 therapy, two CTU reexamination showed the volume of tumor was significantly reduced, and the disease was effectively controlled. Three months after CABG, RC was performed. Postoperative pathological examination revealed high-grade muscle-invasive urothelial carcinoma of the bladder, T2bN0M0, with negative surgical margins. As a conclusion, the neoadjuvant therapy with antibody-drug conjugations represented by RC48 shows a bright future, so it's worth further exploration.
-
Key words:
- bladder cancer /
- neoadjuvant /
- RC 48 /
- antibody-drug conjugate
-

-
[1] Babjuk M, Burger M, Compérat EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer(tat1 and carcinoma in situ)-2019 update[J]. Eur Urol, 2019, 76(5): 639-657. doi: 10.1016/j.eururo.2019.08.016
[2] 中华人民共和国国家卫生健康委员会. 膀胱癌诊疗指南(2022年版)
[3] Nielsen ME, Smith AB, Meyer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006[J]. Cancer, 2014, 120(1): 86-95. doi: 10.1002/cncr.28397
[4] Witjes JA. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines[J]. Eur Urol, 2021, 79: 82-104. doi: 10.1016/j.eururo.2020.03.055
[5] Hanna N, Trinh QD, Seisen T, et al. Effectiveness of neoadjuvant chemotherapy for muscle-invasive bladder cancer in the current real world setting in the USA[J]. Eur Urol Oncol, 2018, 1(1): 83-90. doi: 10.1016/j.euo.2018.03.001
[6] Flaig TW, Spiess PE, Abern M, et al. NCCN GuidelinesⓇ Insights: Bladder Cancer, Version 2.2022[J]. J Natl Compr Canc Netw, 2022, 20(8): 866-878. doi: 10.6004/jnccn.2022.0041
[7] Advanced Bladder Cancer(ABC)Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer(ABC)meta-analysis collaboration[J]. Eur Urol, 2005, 48(2): 202-205. doi: 10.1016/j.eururo.2005.04.006
[8] Dash A, Pettus JA 4th, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience[J]. Cancer, 2008, 113(9): 2471-2477. doi: 10.1002/cncr.23848
[9] Sherif A. Re: Phase Ⅱ trial of neoadjuvant gemcitabine and cisplatin in patients with resectable bladder carcinoma[J]. Int Braz J Urol, 2007, 33(6): 840-841. doi: 10.1590/S1677-55382007000600015
[10] Khaled HM, Shafik HE, Zabhloul MS, et al. Gemcitabine and cisplatin as neoadjuvant chemotherapy for invasive transitional and squamous cell carcinoma of the bladder: effect on survival and bladder preservation[J]. Clin Genitourin Cancer, 2014, 12(5): e233-240. doi: 10.1016/j.clgc.2014.04.002
[11] Niedersüss-Beke D, Puntus T, Kunit T, et al. Neoadjuvant chemotherapy with gemcitabine plus cisplatin in patients with locally advanced bladder cancer[J]. Oncology, 2017, 93(1): 36-42. doi: 10.1159/000463389
[12] Yuh BE, Ruel N, Wilson TG, et al. Pooled analysis of clinical outcomes with neoadjuvant cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer[J]. J Urol, 2013, 189(5): 1682-1686. doi: 10.1016/j.juro.2012.10.120
[13] Pfister, C. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER Trial[J]. J Clin Oncol, 2022, 40: 2013-2022.
[14] Pfister C, Gravis G, Fléchon A, et al. Randomized Phase Ⅲ Trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses[J]. Eur Urol, 2021, 79(2): 214-221. doi: 10.1016/j.eururo.2020.08.024
[15] Chung DY. Comparison of oncologic outcomes of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin(ddMVAC)with Gemcitabine and Cisplatin(GC)as neoadjuvant chemotherapy for muscle-invasive bladder cancer: systematic review and meta-analysis[J]. Cancers(Basel), 2021, doi: 10.3390/cancers13112770(2021).
[16] 中华医学会泌尿外科分会. 中国膀胱癌诊断治疗指南(2021版).
[17] Chang SS. Re: Surgical safety of radical cystectomy and pelvic lymph node dissection following neoadjuvant pembrolizumab in patients with bladder cancer: prospective assessment of perioperative outcomes from the PURE-01 Trial[J]. J Urol, 2020, 204(5): 1094-1095. doi: 10.1097/JU.0000000000001260
[18] Powles T, Kockx M, Rodriguez-Vida A, et al. Publisher Correction: Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial[J]. Nat Med, 2020, 26(6): 983. doi: 10.1038/s41591-020-0923-3
[19] Galsky MD. Phase Ⅲ study of perioperative pembrolizumab(pembro)plus cystectomy versus cystectomy alone in cisplatin-ineligible patients(pts)with muscle-invasive bladder cancer(MIBC): KEYNOTE-905[J]. J Clin Oncology, 2020, 38: TPS593-TPS593.
[20] Hoimes CJ. Phase Ib/Ⅱ neoadjuvant(N-)pembrolizumab(P)and chemotherapy for locally advanced urothelial cancer(laUC): Final results from the cisplatin(C)-eligible cohort of HCRN GU14-188[J]. J Clin Oncology, 2020, 38: 5047-5047. doi: 10.1200/JCO.2020.38.15_suppl.5047
[21] Gupta S. Results from BLASST-1(Bladder Cancer Signal Seeking Trial)of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer(MIBC)undergoing cystectomy[J]. J Clin Oncol, 2020, 38: 439-439.
[22] Cathomas R. Safety and efficacy of perioperative cisplatin/gemcitabine(cis/gem)and durvalumab(durva)for operable muscle-invasive urothelial carcinoma(MIUC): SAKK 06/17[J]. J Clin Oncol, 2021, 39: 430-430.
[23] Galsky MD, Hoimes CJ, Necchi A, et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: Phase Ⅲ KEYNOTE-866 and KEYNOTE-905/EV-303[J]. Future Oncol, 2021, 17(24): 3137-3150. doi: 10.2217/fon-2021-0273
[24] Gao J. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma[J]. Nat Med, 2020, 26: 1845-1851. doi: 10.1038/s41591-020-1086-y
[25] van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial[J]. Nat Med, 2020, 26(12): 1839-1844. doi: 10.1038/s41591-020-1085-z
[26] Siefker-Radtke AO, Necchi A, Park SH, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study[J]. Lancet Oncol, 2022, 23(2): 248-258. doi: 10.1016/S1470-2045(21)00660-4
[27] Franza A, Pirovano M, Giannatempo P, et al. Erdafitinib in locally advanced/metastatic urothelial carcinoma with certain FGFR genetic alterations[J]. Future Oncol, 2022, 18(19): 2455-2464. doi: 10.2217/fon-2021-1151
[28] Chang E, Weinstock C, Zhang L, et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma[J]. Clin Cancer Res, 2021, 27(4): 922-927. doi: 10.1158/1078-0432.CCR-20-2275
[29] Yu EY. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma(EV201): a multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2021, 22: 872-882. doi: 10.1016/S1470-2045(21)00094-2
[30] Powles T. Enfortumab vedotin in previously treated advanced urothelial carcinoma[J]. N Engl J Med, 2021, 384: 1125-1135. doi: 10.1056/NEJMoa2035807
[31] Rosenberg JE. Study EV-103: Preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma[J]. J Clin Oncol, 2020, 38: 441-441.
[32] Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A Phase Ⅱ Open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors[J]. J Clin Oncol, 2021, 39(22): 2474-2485. doi: 10.1200/JCO.20.03489
[33] Sheng X. Open-label, Multicenter, Phase Ⅱ Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma[J]. Clin Cancer Res, 2021, 27: 43-51. doi: 10.1158/1078-0432.CCR-20-2488
[34] Sheng X. An open-label, single-arm, multicenter, phase Ⅱ study of RC48-ADC to evaluate the efficacy and safety of subjects with HER2 overexpressing locally advanced or metastatic urothelial cancer(RC48-C009)[J]. J Clin Oncol, 2021, 39: 4584-4584. doi: 10.1200/JCO.2021.39.15_suppl.4584
[35] Zhou L. Study RC48-C014: Preliminary results of RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma[J]. J Clin Oncol, 2022, 40: 515-515.
[36] Petrylak DP. Study EV-103 Cohort H: Antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients with muscle-invasive bladder cancer who are cisplatin-ineligible[J]. J Clin Oncol, 2022, 40: 4582-4582. doi: 10.1200/JCO.2022.40.16_suppl.4582
[37] 李博乐. 肿瘤抗体药物偶联物的研发进展和挑战[J]. 中国肿瘤临床, 2022, 49: 850-857. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZL202216011.htm
-





 下载:
下载: