-
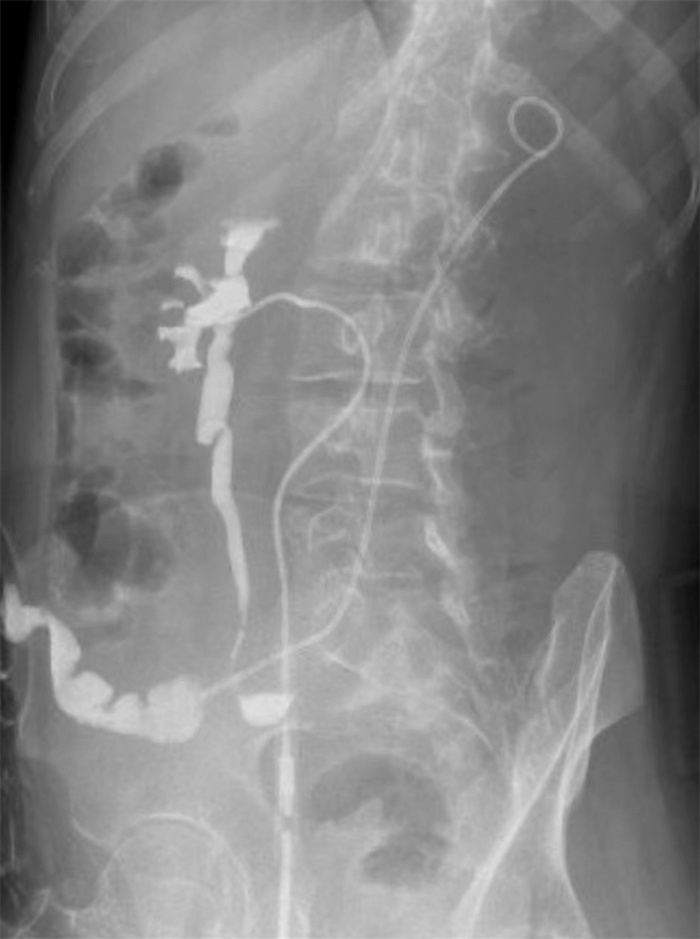
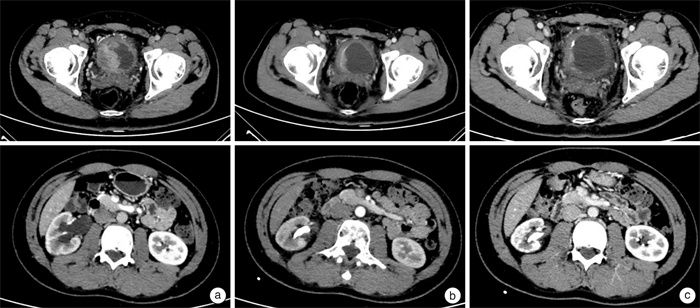
摘要: 本文介绍1例在经过膀胱癌根治术后出现复发的肌层浸润性膀胱癌患者的多学科联合诊疗(MDT)过程。该患者在诊断性电切明确病理诊断后,行新辅助化疗+免疫治疗,病灶出现部分缓解。随后对该患者进行膀胱癌根治术+回肠通道术,术后进行2周期辅助化疗+免疫治疗,后续进行免疫治疗维持,但患者因疲乏、食欲不振中断治疗。后定期复查,于术后1年余开始发现腹膜后淋巴结转移合并肾积水以及骨转移。经MDT团队讨论,在全身系统治疗方面,对该患者再次进行了化疗+免疫治疗,病情再次反复后,改为抗体药物偶联物(ADC)类药物(维迪西妥单抗治疗); 在局部治疗方面,介入放射科进行了顺行输尿管狭窄段球囊扩张+输尿管支架置入,肿瘤科进行了针对骨转移灶的放射治疗,病情得到有效控制。整个诊疗过程体现了MDT团队能够综合动态评估患者病情、快速调整诊治策略的优势,为患者提供了个体化治疗方案并使其获得了更好的生活质量。Abstract: This article introduced the multi-disciplinary treatment(MDT) of a patient with recurrent muscle-invasive bladder cancer. Firstly, the patient underwent neoadjuvant chemotherapy+immunotherapy after confirmed pathological diagnosis, and the lesion showed partial response. Subsequently, the patient underwent radical cystectomy, two cycles of adjuvant chemotherapy+immunotherapy and maintenance immunotherapy were performed thereafter.But the treatment was terminated due to fatigue and anorexia. During routine follow-up, retroperitoneal lymph node metastasis, hydronephrosis and bone metastasis were found about one year after surgery. MDT team worked out some recommendations. In terms of systemic treatment, chemotherapy and immunotherapy were performed again, and after recurrence happened once again, the patient was treated with HER2 ADC(RC48). In terms of local treatment, interventional radiologist carried out balloon dilation of the ureter and ureteral stent placement, and radiation oncologist carried out radiation therapy on bone metastases. Finally, the disease was effectively controlled. The entire treatment process shows the advantages of the MDT team in assessing the patient's condition dynamically and adjusting the treatment strategy efficiently. MDT team could provide the patients with individualized treatment plans and better quality of life.
-
Key words:
- recurrent bladder cancer /
- chemotherapy /
- immunotherapy /
- antibody-drug conjugate /
- radiotherapy
-

-
[1] Sherif A, Holmberg L, Rintala E, et al. Neoadjuvantcisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies[J]. Eur Urol, 2004, 45(3): 297-303. doi: 10.1016/j.eururo.2003.09.019
[2] Lin TX, Li KW, Fan JH, et al. Interim results from a multicenter clinical study of tislelizumab combined with gemcitabine and cisplatin as neoadjuvant therapy for patients with cT2-T4aN0M0 MIBC[J]. J Clin Oncol, 2022, 40(16_suppl): 4580-4580. doi: 10.1200/JCO.2022.40.16_suppl.4580
[3] Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma[J]. N Engl J Med, 2021, 385(9): 864. doi: 10.1056/NEJMx210012
[4] Powles T, Park SH, Caserta C, et al. Avelumab first-line(1 L)maintenance for advanced urothelial carcinoma(UC): Long-term follow-up from the JAVELIN Bladder 100 trial in subgroups defined by 1 L chemotherapy regimen and analysis of overall survival(OS)from start of 1 L chemotherapy[J]. J Clin Oncol, 2023, 41(19): 3486-3492. doi: 10.1200/JCO.22.01792
[5] Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma[J]. N Engl J Med, 2020, 383(13): 1218-1230. doi: 10.1056/NEJMoa2002788
[6] O'Donnell PH, Arkenau HT, Sridhar SS, et al. Patient-reported outcomes and inflammatory biomarkers in patients with locally advanced/metastatic urothelial carcinoma treated with durvalumab in phase 1/2 dose-escalation study 1108[J]. Cancer, 2020, 126(2): 432-443. doi: 10.1002/cncr.32532
[7] Powles T, O'Donnell PH, Massard C, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study[J]. JAMA Oncol, 2017, 3(9): e172411. doi: 10.1001/jamaoncol.2017.2411
[8] Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer(IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2020, 395(10236): 1547-1557. doi: 10.1016/S0140-6736(20)30230-0
[9] Powles T, Csöszi T, Özgüro lu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma(KEYNOTE-361): a randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2021, 22(7): 931-945. doi: 10.1016/S1470-2045(21)00152-2
[10] Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma(DANUBE): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Oncol, 2020, 21(12): 1574-1588. doi: 10.1016/S1470-2045(20)30541-6
[11] Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvantatezolizumab in operable urothelial carcinoma in the ABACUS trial[J]. Nat Med, 2019, 25(11): 1706-1714. doi: 10.1038/s41591-019-0628-7
[12] Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study[J]. Lancet Oncol, 2013, 14(8): 769-776. doi: 10.1016/S1470-2045(13)70162-1
[13] Sridhar SS, Blais N, Tran B, et al. Efficacy and Safety of nab-Paclitaxel vs Paclitaxel on Survival in Patients With Platinum-Refractory Metastatic Urothelial Cancer: The Canadian Cancer Trials Group BL. 12 Randomized Clinical Trial[J]. JAMA Oncol, 2020, 6(11): 1751-1758. doi: 10.1001/jamaoncol.2020.3927
[14] Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy(RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial[J]. Lancet Oncol, 2020, 21(1): 105-120. doi: 10.1016/S1470-2045(19)30668-0
[15] Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma[J]. N Engl J Med, 2019, 381(4): 338-348. doi: 10.1056/NEJMoa1817323
[16] Sheng X, He ZY, Han WQ, et al. An open-label, single-arm, multicenter, phase Ⅱ study of RC48-ADC to evaluate the efficacy and safety of subjects with HER2 overexpressing locally advanced or metastatic urothelial cancer(RC48-C009)[J]. J Clin Oncol, 2021, 39(15_suppl): 4584-4584. doi: 10.1200/JCO.2021.39.15_suppl.4584
[17] Zhou L, Xu HY, Li SM, et al. Study RC48-C014: Preliminary results of RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma[J]. J Clin Oncol, 2022, 40(6_suppl): 515-515. doi: 10.1200/JCO.2022.40.6_suppl.515
[18] Powles T, Rosenberg JE, Sonpavde GP, et al. EnfortumabVedotin in Previously Treated Advanced Urothelial Carcinoma[J]. N Engl J Med, 2021, 384(12): 1125-1135. doi: 10.1056/NEJMoa2035807
[19] Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A Phase Ⅱ Open-Label Study of SacituzumabGovitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors[J]. J Clin Oncol, 2021, 39(22): 2474-2485. doi: 10.1200/JCO.20.03489
[20] Tang Q, Zuo W, Wan C, et al. Comprehensive genomic profiling of upper tract urothelial carcinoma and urothelial carcinoma of the bladder identifies distinct molecular characterizations with potential implications for targeted therapy & immunotherapy[J]. Front Immunol, 2023, 13: 1097730. doi: 10.3389/fimmu.2022.1097730
[21] Jonathan E, Rosenberg, Park SH, et al. BAYOU: A phase Ⅱ, randomized, multicenter, double-blind, study of durvalumab(D)in combination with olaparib(O)for the first-line treatment of platinum-ineligible patients with unresectable, stage Ⅳ urothelial carcinoma(UC)[J]. J Clin Oncol, 2022, 40(6_suppl): 437-437. doi: 10.1200/JCO.2022.40.6_suppl.437
[22] Crabb SJ, Hussain SA, Soulis E, et al. A randomized, double blind, biomarker selected, phase Ⅱ clinical trial of maintenance PARP inhibition following chemotherapy for metastatic urothelial carcinoma(mUC): Final analysis of the ATLANTIS rucaparib arm[J]. J Clin Oncol, 2022, 40(6_suppl): 436-436. doi: 10.1200/JCO.2022.40.6_suppl.436
[23] Vignani F, Tambaro R, Giorgi UD, et al. Randomized phase Ⅱ study of niraparib plus best supportive care(BSC)versus BSC alone as maintenance treatment in patients with advanced urothelial carcinoma(UC)whose disease did not progress after first-line platinum-based chemotherapy(PBCT): The Meet-URO12 trial[J]. J Clin Oncol, 2022, 40(6_suppl): 442-442. doi: 10.1200/JCO.2022.40.6_suppl.442
-

| 引用本文: | 刘磊, 张远鹏, 樊继全, 等. 复发性膀胱癌的多学科联合诊疗及文献复习[J]. 临床泌尿外科杂志, 2023, 38(11): 886-892. doi: 10.13201/j.issn.1001-1420.2023.11.016 |
| Citation: | LIU Lei, ZHANG Yuanpeng, FAN Jiquan, et al. Multi-disciplinary treatment in recurrent bladder cancer and literature review[J]. J Clin Urol, 2023, 38(11): 886-892. doi: 10.13201/j.issn.1001-1420.2023.11.016 |
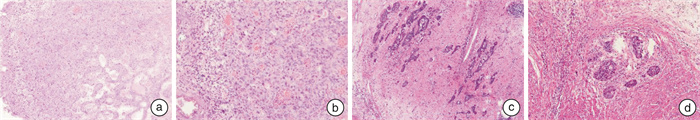
- Figure 1.
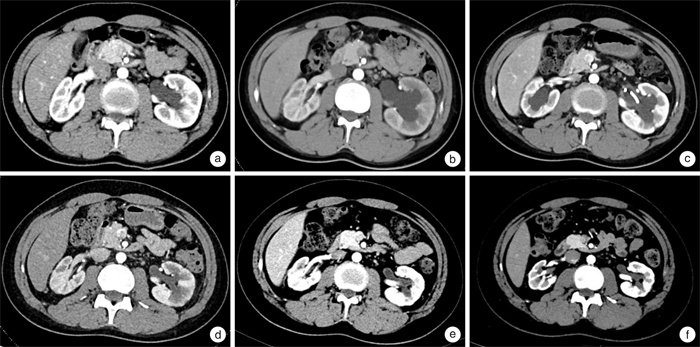
- Figure 2.
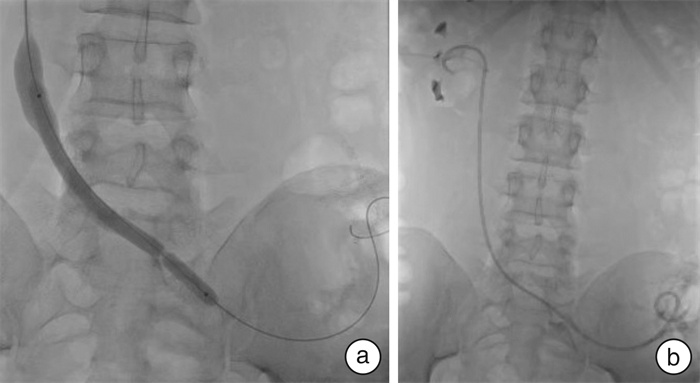
- Figure 3.
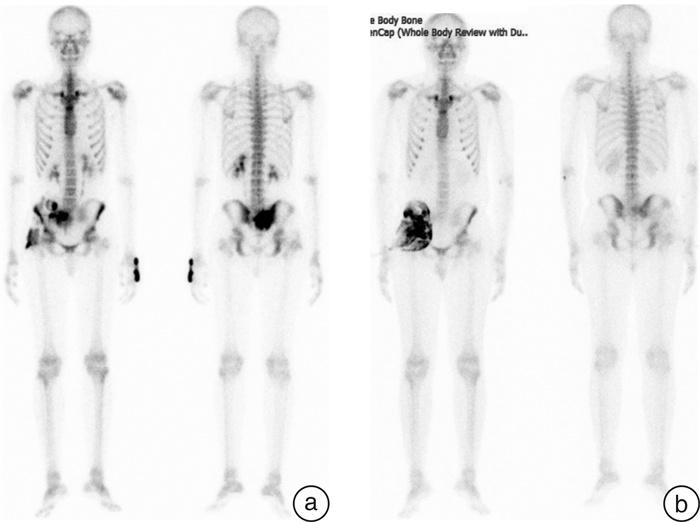
- Figure 4.
- Figure 5.
- Figure 6.



 下载:
下载: