Clinical efficacy of third- and later-line regimen of nivolumab plus statins in the treatment of patients with metastatic renal cell carcinoma and their prognosis factors
-
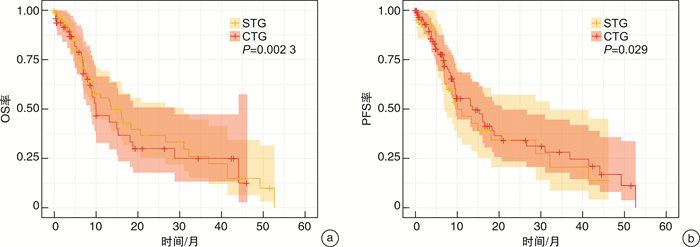
摘要: 目的 探讨纳武单抗联合阿托伐他汀三线及以上方案治疗转移性肾细胞癌(metastatic renal cell carcinoma,mRCC)的临床疗效。 方法 通过回顾性的收集2018年1月—2022年12月在永州市中心医院泌尿外科和肿瘤内科临床二线治疗失败的137例mRCC患者。mRCC患者根据治疗方案分为纳武单抗单药治疗组(single treatment group,STG)和纳武单抗+阿托伐他汀联合治疗组(combined treatment group,CTG)。对所有患者的客观反应率(objective response rate,ORR)、疾病控制率(disease control rate,DCR)、无进展生存期(progression-free survival,PFS)和总生存期(overall survival,OS)进行评估。采用Kaplan-Meier法和log-rank检验对生存资料进行分析。此外,单因素和多因素Cox风险比例回归探讨不同临床特征对PFS和OS的影响。 结果 本研究共纳入137例mRCC患者,其中CTG 46例,STG 91例。CTG和STG的ORR分别为28.26%和20.88%,DCR分别为56.52%和37.36%。CTG的中位PFS和中位OS均高于STG(8.92个月vs 6.80个月,P<0.05;24.22个月vs 10.63个月,P<0.05)。既往未行肾切除、≥2个转移部位和三线以上治疗方案是mRCC患者中位PFS的独立危险因素。而既往未行肾切除和三线以上治疗方案是mRCC患者中位OS的独立危险因素。 结论 纳武单抗联合他汀类药物三线及以上方案治疗mRCC明显优于纳武单抗单药治疗,可显著延长mRCC患者的PFS和OS。有待进一步的前瞻性临床试验验证。Abstract: Objective To evaluate the clinical efficacy of nivolumab plus atorvastatin as a third-or later-line treatment for patients with metastatic renal cell carcinoma(mRCC). Methods A total of 137 patients with mRCC who were histopathologically confirmed and failed in clinical second-line therapy regimens at Yongzhou Center Hospital from January 2018 to December 2022 were retrospectively selected. Patients with mRCC were divided into the single treatment group(STG) of nivolumab monotherapy, and the combined treatment group(CTG) of nivolumab in combination with atorvastatin. The primary outcomes of interest were objective response rate(ORR) and disease control rate(DCR), progression-free survival(PFS), and overall survival(OS). Survival data were analyzed using the Kaplan-Meier method, and the log-rank test was performed. Additionally, Cox proportional hazard regression was used to explore the effect of different clinical features on PFS and OS. Results Of the 137 patients with mRCC included in the research, 91 patients received nivolumab, and 46 patients received nivolumab plus atorvastatin. The ORR of CTG and STG were 28.26% and 20.88%, and the DCR were 56.52% and 37.36%, respectively. The median PFS(mPFS) and median OS(mOS) in CTG were higher than those in the STG(8.92 months vs 6.80 months, P < 0.05; 24.22 months vs 10.63 months, P < 0.05; respectively). No previous nephrectomy, more than two metastatic sites, and >third-line treatment were independent risk factors for the mPFS of patients with mRCC, and no previous nephrectomy and >third-line treatment were independent risk factors for the mOS of patients with mRCC. Conclusion Nivolumab combined with atorvastatin as the third- or later-line regimen greatly prolonged PFS and OS of mRCC patients. A prospective clinical trial is warranted.
-
Key words:
- metastatic renal cell carcinoma /
- nivolumab /
- atorvastatin /
- prognosis
-

-
表 1 mRCC患者临床特征基线期资料
例(%),X±S 项目 总样本(137例) CTG(46例) STG(91例) P值 年龄/岁 62.95±5.69 63.70±5.07 62.57±5.97 0.276 年龄分层 0.355 ≥65岁 49(35.77) 14(30.43) 35(38.46) <65岁 88(64.23) 32(69.57) 56(61.54) 性别 0.575 男 94(68.61) 33(71.74) 61(67.03) 女 43(31.39) 13(28.26) 30(32.97) 肾切除 0.281 是 107(78.10) 37(80.43) 70(76.92) 否 30(21.90) 9(19.57) 21(23.08) 病理类型 0.849 透明细胞 115(83.94) 39(84.78) 76(83.52) 非透明细胞 22(16.06) 7(15.22) 15(16.48) IMDC风险分层 0.876 高危 25(18.25) 9(19.57) 16(17.58) 中危 85(62.04) 29(63.04) 56(61.54) 低危 27(19.71) 8(17.39) 19(20.88) 转移方式 肺转移 74(54.01) 23(50.00) 51(56.04) 0.503 淋巴结转移 66(48.18) 20(43.48) 46(50.55) 0.434 骨转移 42(30.66) 11(23.91) 31(34.07) 0.224 肝转移 23(16.79) 6(13.04) 17(18.68) 0.404 脑转移 6(4.38) 1(2.17) 5(5.49) 0.370 ≥2个转移部位 71(51.82) 23(50.00) 48(52.75) 0.761 二线治疗方案 0.326 帕博利珠单抗+仑伐替尼 17(12.41) 3(6.52) 14(15.38) 帕博利珠单抗+舒尼替尼 14(10.22) 6(13.04) 8(8.79) 帕博利珠单抗+阿昔替尼 18(13.14) 6(13.04) 12(13.19) 特瑞普利单抗+阿昔替尼 12(8.76) 7(15.22) 5(5.49) 替雷利珠单抗+阿昔替尼 22(16.06) 8(17.39) 14(15.38) 信迪利单抗+阿昔替尼 27(19.71) 10(21.74) 17(18.68) 特瑞普利单抗+舒尼替尼 18(13.14) 3(6.52) 15(16.48) 替雷利珠单抗+舒尼替尼 4(2.92) 2(4.35) 2(2.20) 信迪利单抗+舒尼替尼 5(3.65) 1(2.17) 4(4.40) 治疗线数 0.294 三线治疗 81(59.12) 24(52.17) 57(62.64) 三线以上治疗 56(40.88) 22(47.83) 34(37.36) 表 2 mRCC患者治疗反应性
例(%) 治疗反应 CTG(46例) STG(91例) χ2 P值 CR 5(10.87) 7(7.69) PR 8(17.39) 12(13.19) SD 13(28.26) 15(16.48) PD 20(43.48) 53(58.24) ORR 13(28.26) 19(20.88) 1.255 0.263 DCR 26(56.52) 34(37.36) 5.144 0.024 表 3 PFS相关因素的Cox风险比例回归分析
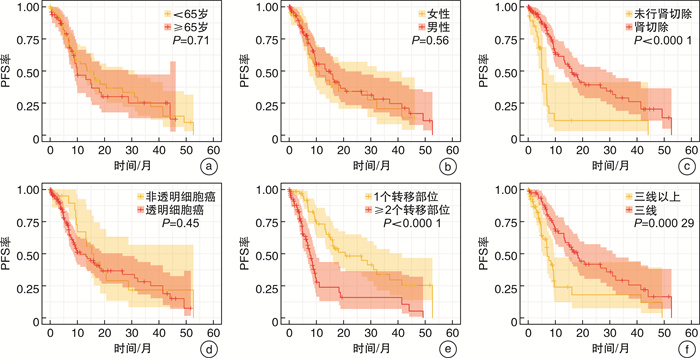
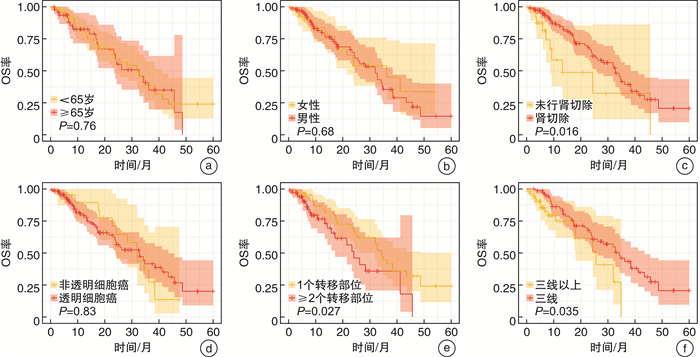
临床特征 单因素Cox回归 多因素Cox回归 OR 95%CI P值 OR 95%CI P值 年龄(≥65岁vs<65岁) 1.097 0.679~1.772 0.706 性别(男vs女) 0.865 0.529~1.415 0.563 既往肾切除(是vs否) 0.278 0.161~0.479 <0.001 0.376 0.214~0.662 0.001 病理类型(透明细胞癌vs非透明细胞癌) 1.263 0.690~2.309 0.449 转移部位(≥2个vs 1个) 2.974 1.837~4.814 <0.001 2.131 1.269~3.579 0.004 治疗线数(三线vs三线以上) 0.418 0.258~0.680 <0.001 0.529 0.318~0.880 0.014 表 4 OS相关因素的Cox风险比例回归分析
临床特征 单因素Cox回归 多因素Cox回归 OR 95%CI P值 OR 95%CI P值 年龄(≥65岁vs<65岁) 1.090 0.631~1.885 0.757 性别(男vs女) 1.129 0.631~2.020 0.683 既往肾切除(是vs否) 0.434 0.216~0.874 0.019 0.449 0.223~0.904 0.025 病理类型(透明细胞癌vs非透明细胞癌) 0.931 0.488~1.775 0.828 转移部位(≥2个vs 1个) 1.871 1.066~3.286 0.029 1.489 0.821~2.698 0.190 治疗线数(三线vs三线以上) 0.518 0.278~0.965 0.038 0.532 0.285~0.994 0.048 -
[1] 赵建华, 郑盛锋, 张明津, 等. LINC00475在肾透明细胞癌中的表达及对肾癌细胞增殖和侵袭的影响[J]. 临床泌尿外科杂志, 2023, 38(10): 785-790. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2023.10.012
[2] 段刘剑, 章顺, 张林, 等. 外周血免疫指标对肾癌术前诊断与分期的临床意义[J]. 临床泌尿外科杂志, 2023, 38(2): 120-123, 127. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2023.02.008
[3] Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14(CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries[J]. Lancet, 2018, 391(10125): 1023-1075. doi: 10.1016/S0140-6736(17)33326-3
[4] Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa inmetastatic renal-cell carcinoma[J]. N Engl J Med, 2007, 356(2): 115-124. doi: 10.1056/NEJMoa065044
[5] Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastaticrenal cell carcinoma: results of a randomized phase Ⅲ trial[J]. J ClinOncol, 2010, 28(6): 1061e8.
[6] Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma[J]. N Engl J Med, 2018, 378(14): 1277-1290. doi: 10.1056/NEJMoa1712126
[7] Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib foradvanced renal-cell carcinoma[J]. N Engl J Med, 2019, 380(12): 1103-1115. doi: 10.1056/NEJMoa1816047
[8] Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma(IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial[J]. Lancet, 2019, 393(10189): 2404-2415. doi: 10.1016/S0140-6736(19)30723-8
[9] Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma[J]. N Engl J Med, 2019, 380(12): 1116-1127. doi: 10.1056/NEJMoa1816714
[10] Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renalcell carcinoma[J]. N Engl J Med, 2021, 384(14): 1289-1300. doi: 10.1056/NEJMoa2035716
[11] Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma[J]. N Engl J Med, 2021, 384(9): 829-841. doi: 10.1056/NEJMoa2026982
[12] Rizzo A, Mollica V, Dall'Olio FG, et al. Quality of life assessment in renal cell carcinoma Phase Ⅱ and Ⅲ clinical trials published between 2010 and 2020: a systematic review[J]. Future Oncol, 2021, 17(20): 2671-2681. doi: 10.2217/fon-2021-0069
[13] 梅云涌. 他汀类药物联合心血管药物治疗冠心病的临床效果及药学观察[J]. 中国实用医药, 2023, 18(5): 101-104. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSSA202305023.htm
[14] Santoni M, Monteiro F, Massari F, et al. Statins and renal cell carcinoma: Antitumor activity and influence on cancer risk and survival[J]. Crit Rev Oncol Hematol, 2022, 176: 103731. doi: 10.1016/j.critrevonc.2022.103731
[15] Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates[J]. Nat Rev Clin Oncol, 2013, 10(11): 625-642. doi: 10.1038/nrclinonc.2013.169
[16] Luo Y, She DL, Xiong H, et al. The Prognostic Effect of Statin Use on Urologic Cancers: An Updated Meta-Analysis of 35 Observational Studies[J]. Medicine(Baltimore), 2015, 94(36): e1523.
[17] Chou YC, Lin CH, Wong CS, et al. Statin use and the risk of renal cell carcinoma: national cohort study[J]. J Investig Med, 2020, 68(3): 776-781. doi: 10.1136/jim-2019-001209
[18] Nayan M, Finelli A, Jewett M, et al. Statin use and kidney cancer outcomes: A propensity score analysis[J]. Urol Oncol, 2016, 34(11): 487. e1-487. e6. doi: 10.1016/j.urolonc.2016.06.007
[19] Nayan M, Punjani N, Juurlink DN, et al. Statin use and kidney cancer survival outcomes: A systematic review and meta-analysis[J]. Cancer Treat Rev, 2017, 52: 105-116. doi: 10.1016/j.ctrv.2016.11.009
[20] Neumann E, Klaiber P, Freitag K, et al. Assessment of concomitant non-oncologic medication in patients with surgically treated renal cell carcinoma: impact on prognosis, cell-cycle progression and proliferation[J]. J Cancer Res Clin Oncol, 2019, 145(7): 1835-1843. doi: 10.1007/s00432-019-02914-2
[21] Hamilton RJ, Morilla D, Cabrera F, et al. The associationbetween statin medication and progression after surgery forlocalized renal cell carcinoma[J]. J Urol, 2014, 191(4): 914-919. doi: 10.1016/j.juro.2013.10.141
[22] McKay RR, Lin X, Albiges L, et al. Statins and survival outcomes in patients with metastatic renal cell carcinoma[J]. Eur J Cancer, 2016, 52: 155-162. doi: 10.1016/j.ejca.2015.10.008
[23] Fiala O, Ostašov P, Rozsypalová A, et al. Impact of Concomitant Cardiovascular Medication on Survival of Metastatic Renal Cell Carcinoma Patients Treated with Sunitinib or Pazopanib in the First Line[J]. Target Oncol, 2021, 16(5): 643-652. doi: 10.1007/s11523-021-00829-y
[24] Santoni M, Massari F, Matrana M R, et al. Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma[J]. Eur J Cancer, 2022, 172: 191-198. doi: 10.1016/j.ejca.2022.04.035
[25] Merino Salvador M, Gómez de Cedrón M, Moreno Rubio J, et al. Lipid metabolism and lung cancer[J]. Crit Rev Oncol Hematol, 2017, 112: 31-40. doi: 10.1016/j.critrevonc.2017.02.001
[26] 刘坤, 滕立臣. 肾癌发生发展及转移中脂肪代谢的最新研究进展[J]. 现代肿瘤医学, 2020, 28(23): 4176-4180. https://www.cnki.com.cn/Article/CJFDTOTAL-SXZL202023033.htm
[27] Horiguchi A, Sumitomo M, Asakuma J, et al. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis[J]. Clin Cancer Res, 2004, 10(24): 8648-8655. doi: 10.1158/1078-0432.CCR-04-1568
[28] Porta C, Moroni M, Nastasi G, et al. Hypocholesterolemia and acute myeloid leukemia(AML)[J]. Haematologica, 1991, 76(4): 348.
[29] Ishihara H, Takagi T, Kondo T, et al. Comparable efcacy and safety between second-line and later-line nivolumab therapy for metastatic renal cell carcinoma[J]. Int J Clin Oncol, 2020, 25(4): 705-712. doi: 10.1007/s10147-019-01605-9
[30] Santoni M, Molina-Cerrillo J, Myint ZW, et al. Concomitant Use of Statins, Metformin, or Proton Pump Inhibitors in Patients with Advanced Renal Cell Carcinoma Treated with First-Line Combination Therapies[J]. Target Oncol, 2022, 17(5): 571-581. doi: 10.1007/s11523-022-00907-9
-




 下载:
下载: