-
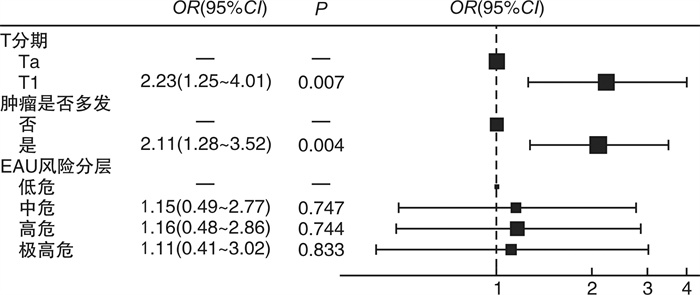
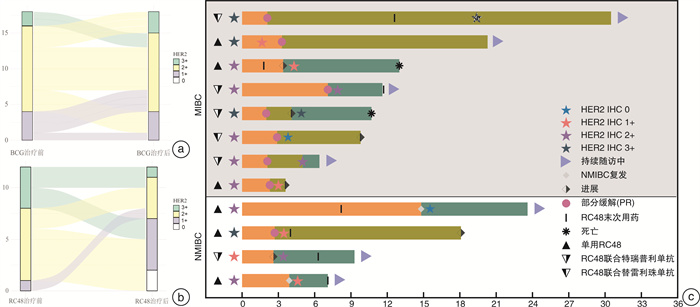
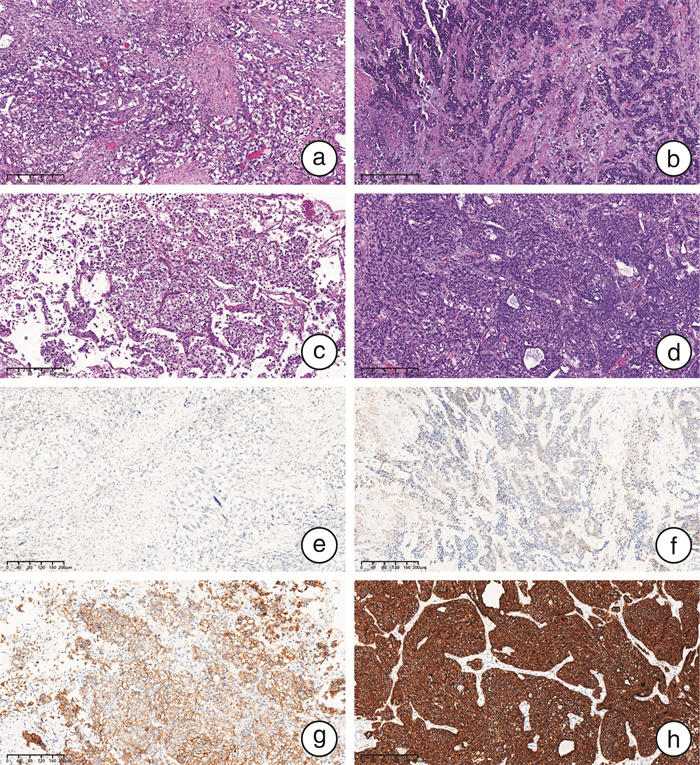
摘要: 目的 探究人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)在膀胱尿路上皮癌中的表达及临床意义。方法 收集2021年4月—2023年4月于四川大学华西医院接受手术治疗的497例膀胱尿路上皮癌患者的临床资料以及手术病理标本HER2蛋白的表达情况,并通过前瞻性队列分析HER2的表达与临床特征和预后的相关性。结果 本研究共纳入497例膀胱尿路上皮癌患者,其中非肌层浸润性膀胱癌(non-muscle-invasive bladder cancer,NMIBC)患者324例,肌层浸润性膀胱癌(muscle-invasive bladder cancer,MIBC)患者173例。NMIBC总体HER2阳性率为52.8%,MIBC总体HER2阳性率为49.1%,2组比较差异无统计学意义(P=0.44)。NMIBC中T1期HER2阳性率(63.1%,99/157)显著高于Ta期(43.1%,72/167)(P < 0.05)。在NMIBC和MIBC中均观察到不同组织亚型间HER2表达情况的显著差异(P < 0.05):HER2阳性率在微乳头型中分别为100%(4/4,NMIBC)和80.0%(8/10,MIBC),在伴鳞状分化亚型中分别为26.7%(4/15,NMIBC)和25.8%(8/31,MIBC);在伴腺样分化亚型中分别为37.5%(3/8,NMIBC)和64.3%(9/14,MIBC)。多因素logistic回归分析结果显示,在NMIBC中T分期以及肿瘤多发是HER2表达阳性的独立风险因素,差异有统计学意义(P < 0.05)。共12例患者接受了靶向HER2的抗体-药物偶联物RC-48治疗,其中58.3%(7/12)的患者治疗后由HER2阳性转为阴性,25.0%(3/12)的患者HER2表达水平与用药前一致,8.3%(1/12)的患者用药后HER2表达水平由1+转为2+。结论 NMIBC HER2表达情况与肿瘤T分期以及肿瘤是否多发相关,但与病理分级、EAU风险评分、肿瘤部位、是否伴原位癌、是否侵犯输尿管等无关;MIBC HER2表达情况与肿瘤组织学亚型相关,与T分期、是否淋巴结转移、是否合并脉管内癌栓、是否神经侵犯等无关。
-
关键词:
- 膀胱尿路上皮癌 /
- 人表皮生长因子受体2 /
- 免疫组织化学
Abstract: Objective To investigate the expression and clinical significance of human epidermal growth factor receptor 2(HER2) in bladder urothelial carcinoma.Methods Clinical data and surgical pathological specimens were collected from 497 bladder urothelial carcinoma patients who underwent surgery at West China Hospital, Sichuan University, from April 2021 to April 2023. The expression of HER2 protein was analyzed, and a prospective cohort analysis was conducted to explore the correlation between HER2 expression and clinical characteristics as well as prognosis.Results A total of 497 bladder urothelial carcinoma patients were included in the study, among which 324 had non-muscle-invasive bladder cancer(NMIBC) and 173 had muscle-invasive bladder cancer(MIBC). The overall HER2 positivity rate was 52.8% in NMIBC and 49.1% in MIBC, with no statistically significant difference between the two groups(P=0.44). In NMIBC, the HER2 positivity rate in T1 stage(63.1%, 99/157) was significantly higher than that in Ta stage(43.1%, 72/167)(P < 0.05). Significant differences in HER2 expression were observed among different histological subtypes in both NMIBC and MIBC(P < 0.05): the HER2 positivity rate was 100%(4/4) in the micropapillary subtype of NMIBC and 80.0%(8/10) in MIBC, 26.7%(4/15) in the squamous differentiation of NMIBC and 25.8%(8/31) in MIBC, and 37.5%(3/8) in the glandular differentiation of NMIBC and 64.3%(9/14) in MIBC. Multivariate logistic regression analysis indicated that in NMIBC, T stage and tumor multiplicity were independent risk factors in HER2 positivity, with statistically significant differences(P < 0.05). A total of 12 patients received the HER2-targeted antibody-drug conjugate RC-48 treatment; among these, 58.3%(7/12) converted from HER2-positive to negative after treatment, 25%(3/12) had consistent HER2 expression levels before and after treatment, and 8.3%(1/12) converted from HER2 1+ to 2+.Conclusion In NMIBC, HER2 expression is associated with tumor T stage and tumor multiplicity, but not with pathological grade, EAU risk score, tumor location, presence of carcinoma in situ, or ureteral involvement. In MIBC, HER2 expression is associated with tumor histological subtype, but not with T stage, lymph node metastasis, vascular invasion, or neural invasion. -

-
表 1 MIBC患者中HER2表达水平与临床及病理资料的关系
例(%),M(P25,P75) 临床及病理因素 总例数(173例) HER2表达水平 P值 阴性(88例) 阳性(85例) 性别 0.351 男 145(83.8) 71(80.7) 74(87.1) 女 28(16.2) 17(19.3) 11(12.9) 年龄 1.000 < 70岁 92(53.2) 47(53.4) 45(52.9) ≥70岁 81(46.8) 41(46.6) 40(47.1) BMI/(kg/m2) 23.1(21.0,24.9) 23.3(21.0,24.7) 23.0(20.8,25.0) 0.825 T分期 0.581 T2 116(67.1) 57(64.8) 59(69.4) T3 34(19.6) 20(22.7) 14(16.5) T4 23(13.3) 11(12.5) 12(14.1) 是否行膀胱全切 0.318 否 90(52.0) 42(47.7) 48(56.5) 是 83(48.0) 46(52.3) 37(43.5) 病理亚型 0.006 无 105(60.7) 44(50.0) 61(71.8) 有 68(39.3) 44(50.0) 24(28.2) 脉管癌栓 1.000 无 138(79.8) 70(79.5) 68(80.0) 有 35(20.2) 18(20.5) 17(20.0) 淋巴结转移 1.000 无 151(87.3) 77(87.5) 74(87.1) 有 22(12.7) 11(12.5) 11(12.9) 神经侵犯 0.096 无 155(89.6) 75(85.2) 80(94.1) 有 18(10.4) 13(14.8) 5(5.9) 术前新辅助治疗 1.000 否 149(86.1) 76(86.4) 73(85.9) 是 24(13.9) 12(13.6) 12(14.1) 高血压病史 0.977 无 125(72.3) 63(71.6) 62(72.9) 有 48(27.7) 25(28.4) 23(27.1) 糖尿病史 0.760 无 143(82.7) 74(84.1) 69(81.2) 有 30(17.3) 14(15.9) 16(18.8) 冠心病史 0.813 无 161(93.1) 81(92.0) 80(94.1) 有 12(6.9) 7(8.0) 5(5.9) 吸烟史 1.000 无 134(77.5) 68(77.3) 66(77.6) 有 39(22.5) 20(22.7) 19(22.4) 饮酒史 0.368 无 143(82.7) 70(79.5) 73(85.9) 有 30(17.3) 18(20.5) 12(14.1) 表 2 NMIBC患者中HER2表达水平与临床与病理资料的关系
例(%),M(P25,P75) 临床及病理因素 总例数(324例) HER2表达水平 P值 阴性(153例) 阳性(171例) 性别 0.872 男 248(76.5) 116(75.8) 132(77.2) 女 76(23.5) 37(24.2) 39(22.8) 年龄 0.433 < 70岁 201(62.0) 91(59.5) 110(64.3) ≥70岁 123(38.0) 62(40.5) 61(35.7) BMI/(kg/m2) 23.4(21.5,25.8) 23.4(21.6,25.7) 23.3(21.5,26.0) 0.902 Hb/(g/L) 135(122,147) 137(121,149) 135(123,146) 0.781 肿瘤大小 0.079 < 3 cm 190(58.6) 98(64.1) 92(53.8) ≥3 cm 134(41.4) 55(35.9) 79(46.2) 分期 < 0.001 Ta 167(51.5) 95(62.1) 72(42.1) T1 157(48.5) 58(37.9) 99(57.9) 亚型 0.107 无 292(90.1) 135(88.2) 157(91.8) 鳞状分化 15(4.6) 11(7.2) 4(2.3) 其他 17(5.2) 7(4.6) 10(5.9) 高血压病史 0.678 无 192(59.3) 93(60.8) 99(57.9) 有 132(40.7) 60(39.2) 72(42.1) 肿瘤是否多发 0.001 否 126(38.9) 74(48.4) 52(30.4) 是 198(61.1) 79(51.6) 119(69.6) 糖尿病史 0.944 无 261(80.6) 124(81.0) 137(80.1) 有 63(19.4) 29(19.0) 34(19.9) 冠心病史 0.261 无 299(92.3) 138(90.2) 161(94.2) 有 25(7.7) 15(9.8) 10(5.8) 吸烟史 0.695 无 265(81.8) 127(83.0) 138(80.7) 有 59(18.2) 26(17.0) 33(19.3) 饮酒史 0.794 无 276(85.2) 129(84.3) 147(86.0) 有 48(14.8) 24(15.7) 24(14.0) EAU2021风险分层 0.012 低危 40(12.3) 27(17.6) 13(7.6) 中危 71(21.9) 38(24.8) 33(19.3) 高危 132(40.7) 56(36.6) 76(44.4) 极高危 81(25.0) 32(20.9) 49(28.7) 侵犯输尿管 0.564 否 229(70.7) 111(72.5) 118(69.0) 是 95(29.3) 42(27.5) 53(31.0) 脉管癌栓 0.109 无 314(96.9) 151(98.7) 163(95.3) 有 10(3.1) 2(1.3) 8(4.7) 伴原位癌 0.207 否 307(94.8) 148(96.7) 159(93.0) 是 17(5.2) 5(3.3) 12(7.0) 既往尿路上皮癌病史 0.799 无 175(54.0) 81(52.9) 94(55.0) 有 149(46.0) 72(47.1) 77(45.0) 表 3 MIBC各病理亚型HER2表达情况
例(%) 项目 例数 阴性(0/1+) 阳性(2+/3+) P值 单纯尿路上皮癌 103 44(42.7) 59(57.3) — 伴病理亚型 74 45(60.8) 29(39.2) 0.001 鳞状分化 31 23(74.2) 8(25.8) 腺样分化 14 5(35.7) 9(64.3) 微乳头样 10 2(20.0) 8(80.0) 肉瘤样 8 7(87.5) 1(12.5) 巢状变异 1 1(100.0) 0(0) 神经内分泌 5 5(100.0) 0(0) 浆细胞样 5 2(40.0) 3(60.0) 表 4 NMIBC各病理亚型HER2表达情况
例(%) 项目 例数 阴性(0/1+) 阳性(2+/3+) P值 单纯尿路上皮癌 292 135(46.2) 157(53.8) — 伴病理亚型 34 21(61.8) 13(38.2) 0.013 鳞状分化 15 11(73.3) 4(26.7) 腺样分化 8 5(62.5) 3(37.5) 微乳头样 4 0(0) 4(100.0) 肉瘤样分化 2 2(100.0) 0(0) 巢状变异 3 3(100.0) 0(0) 其他 2 0(0) 2(100.0) -
[1] Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022[J]. Zhonghua Zhong Liu Za Zhi, 2024, 46(3): 221-231.
[3] Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer(ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2010, 376(9742): 687-697. doi: 10.1016/S0140-6736(10)61121-X
[4] Tan XL, Liu ZC, Cai TN, et al. Prognostic significance of her2 expression in patients with Bacillus Calmette-Guérin-exposed Non-muscle-invasive bladder cancer[J]. Eur Urol Oncol, 2024, 7(4): 760-769. doi: 10.1016/j.euo.2023.10.003
[5] Sheng XN, Wang L, He ZS, et al. Efficacy and safety of disitamab vedotin in patients with human epidermal growth factor receptor 2-positive locally advanced or metastatic urothelial carcinoma: a combined analysis of two phase Ⅱ clinical trials[J]. J Clin Oncol, 2024, 42(12): 1391-1402. doi: 10.1200/JCO.22.02912
[6] 张孟尼, 龚静, 陈雪芹, 等. 尿路上皮癌429例HER2表达情况及临床病理学分析[J]. 中华病理学杂志, 2023, 52(3): 243-249.
[7] 黄世旺, 贾凯鹏, 张天晓, 等. 836例尿路上皮癌人表皮生长因子受体2表达情况及临床病理学分析[J/OL]. 泌尿外科杂志(电子版), 2024, 16(2): 1-6.
[8] 中国抗癌协会肿瘤病理专业委员会, 中国临床肿瘤学会尿路上皮癌专家委员会. 中国尿路上皮癌人表皮生长因子受体2检测临床病理专家共识[J]. 中华肿瘤杂志, 2021, 43(10): 1001-1006.
[9] Sylvester RJ, Rodríguez O, Hernández V, et al. European association of urology(eau)prognostic factor risk groups for Non-muscle-invasive bladder cancer(nmibc)incorporating the who 2004/2016 and who 1973 classification systems for grade: an update from the eau nmibc guidelines panel[J]. Eur Urol, 2021, 79(4): 480-488. doi: 10.1016/j.eururo.2020.12.033
[10] Graus-Porta D, Beerli RR, Daly JM, et al. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling[J]. EMBO J, 1997, 16(7): 1647-1655. doi: 10.1093/emboj/16.7.1647
[11] Hayes DF. HER2 and breast cancer-A phenomenal success story[J]. N Engl J Med, 2019, 381(13): 1284-1286. doi: 10.1056/NEJMcibr1909386
[12] Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network[J]. Nat Rev Mol Cell Biol, 2001, 2(2): 127-137. doi: 10.1038/35052073
[13] Yoon J, Oh DY. HER2-targeted therapies beyond breast cancer—an update[J]. Nat Rev Clin Oncol, 2024. http://www.nature.com/articles/s41571-024-00924-9
[14] Oh DY, Bang YJ. HER2-targeted therapies—a role beyond breast cancer[J]. Nat Rev Clin Oncol, 2020, 17: 33-48. doi: 10.1038/s41571-019-0268-3
[15] Mitri Z, Constantine T, O'Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy[J]. Chemother Res Pract, 2012, 2012: 743193.
[16] Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2024[J]. Journal of the National Comprehensive Cancer Network, 2024, 22(5): 331-357. doi: 10.6004/jnccn.2024.0035
[17] Hong XW, Chen X, Wang HJ, et al. A HER2-targeted antibody-drug conjugate, RC48-ADC, exerted promising antitumor efficacy and safety with intravesical instillation in preclinical models of bladder cancer[J]. Adv Sci, 2023, 10(32): e2302377. doi: 10.1002/advs.202302377
[18] Yan M, Schwaederle M, Arguello D, et al. HER2 expression status in diverse cancers: review of results from 37, 992 patients[J]. Cancer Metastasis Rev, 2015, 34(1): 157-164. doi: 10.1007/s10555-015-9552-6
[19] Meric-Bernstam F, Hainsworth J, Bose R, et al. MyPathway HER2 basket study: Pertuzumab(P)+ trastuzumab(H)treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors[J]. J Clin Oncol, 2021, 39(15_suppl): 3004. doi: 10.1200/JCO.2021.39.15_suppl.3004
[20] Oudard S, Culine S, Vano Y, et al. Multicentre randomised phase Ⅱ trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2[J]. Eur J Cancer, 2015, 51(1): 45-54. doi: 10.1016/j.ejca.2014.10.009
[21] Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase Ⅱ National Cancer Institute trial[J]. J Clin Oncol, 2007, 25(16): 2218-2224. doi: 10.1200/JCO.2006.08.0994
[22] Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2-and HER3-mutant cancers[J]. Nature, 2018, 554(7691): 189-194. doi: 10.1038/nature25475
[23] Font A, Real FX, Puente J, et al. 920TiP Afatinib in patients with advanced or metastatic urothelial carcinoma(UC)with genetic alterations in ErbB receptors 1-3 who failed on platinum-based chemotherapy: The Phase Ⅱ LUX-Bladder 1 trial[J]. Ann Oncol, 2017, 28: v326.
[24] Tang SJ, Dorff TB, Tsao-Wei DD, et al. Single arm phase Ⅱ study of docetaxel and lapatinib in metastatic urothelial cancer: USC trial 4B-10-4[J]. J Clin Oncol, 2016, 34(2_suppl): 424. doi: 10.1200/jco.2016.34.2_suppl.424
[25] Cerbone L, Sternberg CN, Sengeløv L, et al. Results from a phase Ⅰ study of lapatinib with gemcitabine and cisplatin in advanced or metastatic bladder cancer: EORTC trial 30061[J]. Oncology, 2016, 90(1): 21-28. doi: 10.1159/000440959
[26] Tang XR, Chen ZY, Thomas J, et al. Abstract 472: HER2 as a therapeutic target in bladder cancer[J]. Cancer Res, 2023, 83(7_Supplement): 472. doi: 10.1158/1538-7445.AM2023-472
[27] Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer[J]. Cell, 2017, 171(3): 540-556. e25. doi: 10.1016/j.cell.2017.09.007
[28] Moktefi A, Pouessel D, Liu J, et al. Reappraisal of HER2 status in the spectrum of advanced urothelial carcinoma: a need of guidelines for treatment eligibility[J]. Mod Pathol, 2018, 31(8): 1270-1281. doi: 10.1038/s41379-018-0023-9
[29] Zhou L, Shao ZT, Liu YQ, et al. HER2 expression associated with clinical characteristics and prognosis of urothelial carcinoma in a Chinese population[J]. Oncologist, 2023, 28(8): e617-e624. doi: 10.1093/oncolo/oyad070
[30] Hayashi T, Seiler R, Oo HZ, et al. Targeting HER2 with T-DM1, an antibody cytotoxic drug conjugate, is effective in HER2 over expressing bladder cancer[J]. J Urol, 2015, 194(4): 1120-1131. doi: 10.1016/j.juro.2015.05.087
[31] Miligy IM, Badr N, Stevens A, et al. Pathological changes following neoadjuvant endocrine therapy(NAET): a multicentre study of 391 breast cancers[J]. Int J Mol Sci, 2024, 25(13): 7381. doi: 10.3390/ijms25137381
-

| 引用本文: | 周海鹏, 林天海, 张孟尼, 等. HER2在膀胱尿路上皮癌中的表达情况及临床意义[J]. 临床泌尿外科杂志, 2024, 39(9): 769-776. doi: 10.13201/j.issn.1001-1420.2024.09.004 |
| Citation: | ZHOU Haipeng, LIN Tianhai, ZHANG Mengni, et al. Expression of HER2 in bladder urothelial carcinoma and its clinical significance[J]. J Clin Urol, 2024, 39(9): 769-776. doi: 10.13201/j.issn.1001-1420.2024.09.004 |
- Figure 1.
- Figure 2.
- Figure 3.



 下载:
下载: