Safety and short-term efficacy of chemotherapy combined with PD-1 inhibitors in the treatment of metastatic bladder cancer
-
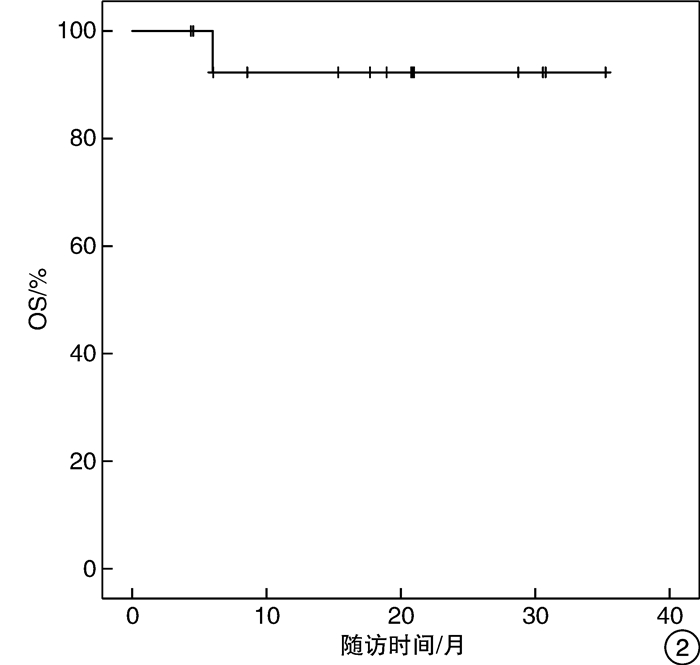
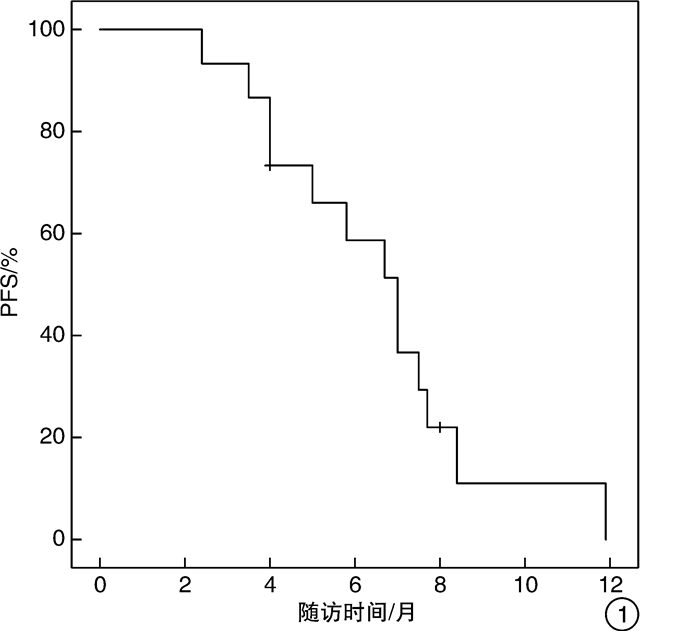
摘要: 目的 评价化疗联合PD-1抑制剂治疗转移性膀胱癌的初步疗效及安全性。方法 回顾性分析2020年5月—2021年5月就诊于北京市朝阳区桓兴肿瘤医院行化疗联合PD-1抑制剂治疗的14例转移性膀胱癌患者的临床资料,病理均为尿路上皮癌。治疗方案为静脉滴注国产PD-1抑制剂(信迪利单抗200 mg、特瑞普利单抗240 mg、替雷利珠单抗200 mg)每3周1次,应用吉西他滨+顺铂(Cisplatin+Gemcitabine,GC)方案或顺铂不耐受时应用吉西他滨+卡铂或白蛋白紫杉醇+卡铂,分析客观缓解率、无进展生存期及不良反应。结果 14例患者中位年龄为64(46.0~82.0)岁,客观缓解率为57.14%(8/14),疾病控制率为92.86%(13/14)。主要不良反应中转氨酶及胆红素升高1例(7.14%),甲状腺功能减退症1例(7.14%),甲状腺功能亢进1例(7.14%),皮疹3例(21.43%),胃肠道反应(恶心伴或不伴呕吐)3例(21.43%),脱发1例(7.14%),骨髓抑制2例(14.29%),乏力1例(7.14%),肾功能损伤1例(7.14%)。患者的不良反应主要为1~2级,3例发生3~4级不良反应,经对症治疗好转。结论 化疗联合PD-1抑制剂治疗转移性膀胱癌有较高的客观缓解率,尤其是GC方案联合PD-1抑制剂,且不良反应多可耐受,为临床抉择提供参考。Abstract: Objective To investigate the preliminary efficacy and safety of chemotherapy combined with PD-1 inhibitors in the treatment of metastatic bladder cancer.Methods The clinical data of 14 patients with metastatic bladder cancer who received chemotherapy combined with PD-1 inhibitor in Cancer Hospital of HuanXing Chaoyang District Beijing from May 2020 to May 2021 were retrospectively analyzed, all with uroepithelial carcinoma on pathology. All patients received infusion of domestic PD-1 inhibitors (200 mg Sintilimab, 240 mg Toripalimab or 200 mg Tislelizumab intravenously every 3 weeks, and gemcitabine + cisplatin (GC) regimen was applied or gemcitabine + carboplatin and albumin paclitaxel + carboplatin were applied when cisplatin was intolerant. The objective remission rate (ORR), progression-free survival (PFS), and adverse effects were analyzed.Results The median age was 64 (46.0-82.0) years, with an ORR of 57.14% (8/14) and disease control rate (DCR) of 92.86% (13/14) in 14 patients. The main adverse effects included elevated transaminases and bilirubin (n=1; 7.14%), hypothyroidism (n=1; 7.14%), hyperthyroidism (n=1; 7.14%), the rash (n=3; 21.43%), gastrointestinal reactions (nausea with or without vomiting) (n=3; 21.43%), hair loss (n=1; 7.14%), bone marrow suppression (n=2; 14.29%), fatigue (n=1; 7.14%) and renal impairment (n=1; 7.14%). Most adverse effects were of grade 1-2, and grade 3-4 adverse events occurred in three cases, which improved with symptomatic treatment.Conclusion Chemotherapy combined with PD-1 inhibitors in the treatment of metastatic bladder cancer has a high ORR, especially GC combined with PD-1 inhibitors, and most of the adverse reactions can be tolerated. It provides reference for clinical decision.
-
Key words:
- PD-1 inhibitor /
- chemotherapy /
- metastatic bladder cancer
-

-
表 1 患者不良反应情况
例 项目 分级 总发生率%(1~4级) 0 1 2 3 4 白细胞减少 13 0 0 0 1 7.14 中性粒细胞减少 13 0 0 0 1 7.14 血红蛋白减少 11 1 2 0 0 21.43 消化道反应 11 1 2 0 0 21.43 肝功能损伤 13 0 0 1 0 7.14 肾功能损伤 13 1 0 0 0 7.14 皮疹 11 2 1 0 0 21.43 脱发 12 2 0 0 0 14.29 乏力 13 1 0 0 0 7.14 甲状腺功能减退 13 1 0 0 0 7.14 甲状腺功能亢进 13 1 0 0 0 7.14 -
[1] Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. doi: 10.3322/caac.21654
[2] 郑荣寿, 孙可欣, 张思维, 等. 2015年中国恶性肿瘤流行情况分析[J]. 中华肿瘤杂志, 2019, 41(1): 19-28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005
[3] Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer[J]. Eur Urol, 2017, 71(3): 462-475. doi: 10.1016/j.eururo.2016.06.020
[4] Flaig TW, Spiess PE, Agarwal N et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2020, 18(3): 329-354. doi: 10.6004/jnccn.2020.0011
[5] Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines[J]. Eur Urol, 2021, 79(1): 82-104. doi: 10.1016/j.eururo.2020.03.055
[6] Chism DD. Urothelial Carcinoma of the Bladder and the Rise of Immunotherapy[J]. J Natl Compr Canc Netw, 2017, 15(10): 1277-1284. doi: 10.6004/jnccn.2017.7036
[7] Siefker-Radtke A, Curti B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition[J]. Nat Rev Urol, 2018, 15(2): 112-124. doi: 10.1038/nrurol.2017.190
[8] Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma(IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial[J]. Lancet, 2018, 391(10122): 748-757. doi: 10.1016/S0140-6736(17)33297-X
[9] Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma[J]. N Engl J Med, 2017, 376(11): 1015-1026. doi: 10.1056/NEJMoa1613683
[10] Rosenberg JE, Hoffman-censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial[J]. Lancet, 2016, 387(10031): 1909-1920. doi: 10.1016/S0140-6736(16)00561-4
[11] Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy(CheckMate 275): a multicentre, single-arm, phase 2 trial[J]. Lancet Oncol, 2017, 18(3): 312-322. doi: 10.1016/S1470-2045(17)30065-7
[12] Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure(JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial[J]. Lancet Oncol, 2018, 19(1): 51-64. doi: 10.1016/S1470-2045(17)30900-2
[13] Ye D, Liu J, Zhou A, et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma[J]. Cancer Sci, 2021, 112(1): 305-313. doi: 10.1111/cas.14681
[14] Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial[J]. Lancet, 2017, 389(10064): 67-76. doi: 10.1016/S0140-6736(16)32455-2
[15] Vuky J, Balar AV, Castellano D, et al. Long-Term Outcomes in KEYNOTE-052: Phase Ⅱ Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer[J]. J Clin Oncol, 2020, 38(23): 2658-2666. doi: 10.1200/JCO.19.01213
[16] Galsky MD, JÁA A, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer(IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2020, 395(10236): 1547-1557. doi: 10.1016/S0140-6736(20)30230-0
[17] Powles T, Csöszi T, Özgüroǧlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma(KEYNOTE-361): a randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2021, 22(7): 931-945. doi: 10.1016/S1470-2045(21)00152-2
[18] von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase Ⅲ study[J]. J Clin Oncol, 2000, 18(17): 3068-3077. doi: 10.1200/JCO.2000.18.17.3068
-




 下载:
下载: