Gemcitabine and epirubicin infusion chemotherapy for non-muscle invasive bladder cancer: a systematic review and meta analysis
-
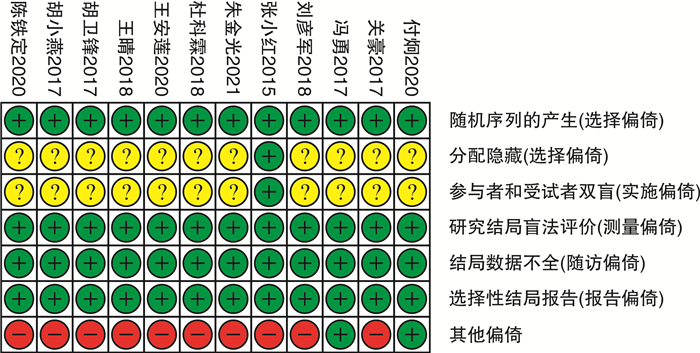
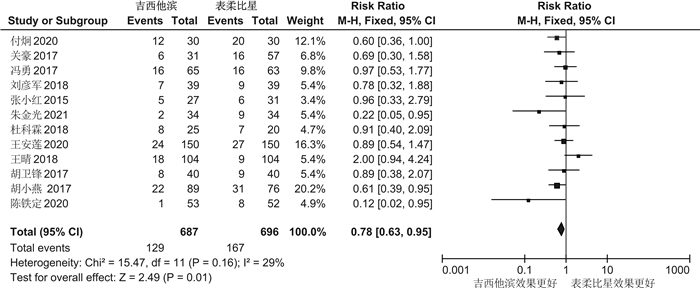
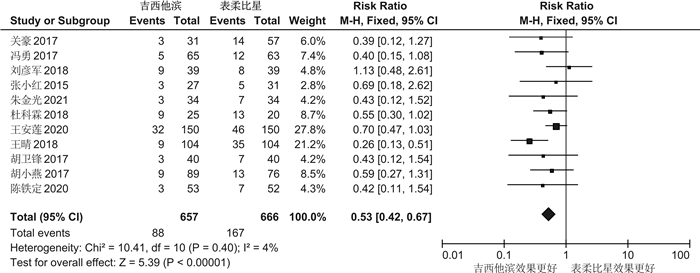
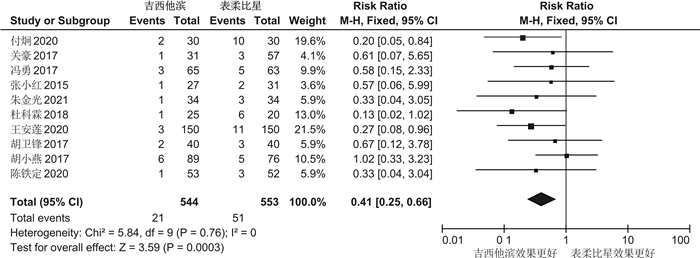
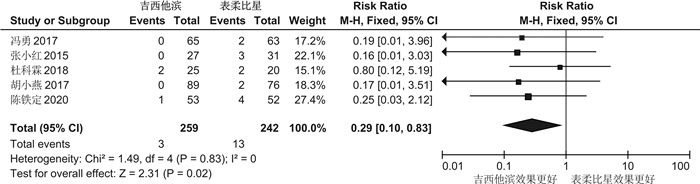
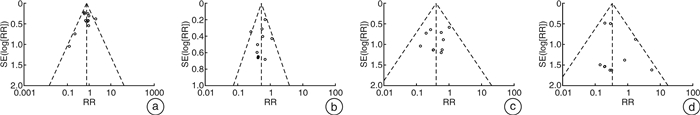
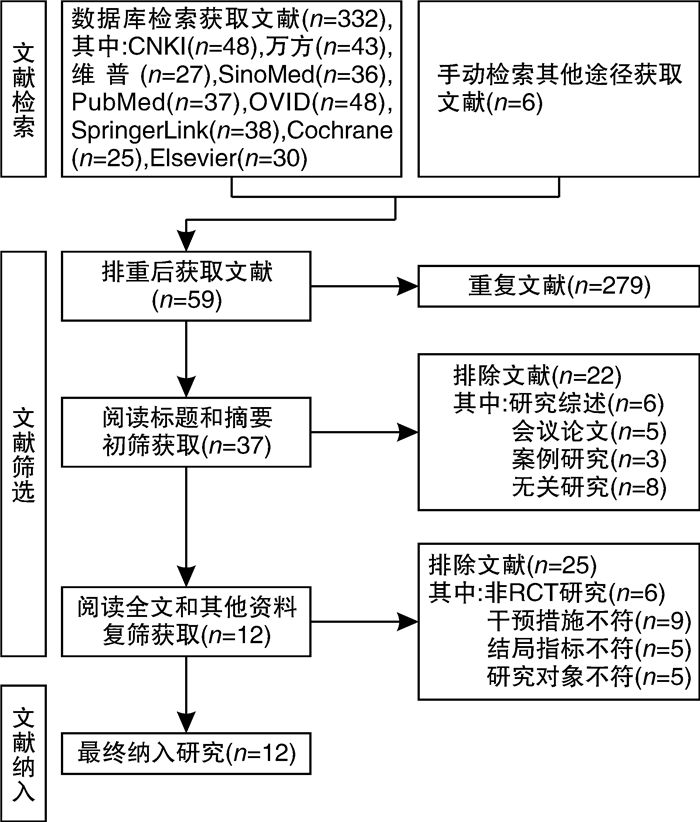
摘要: 目的 系统评价吉西他滨(GEM)和表柔比星(EPI)对非肌层浸润性膀胱癌(NMIBC)患者灌注化疗后有效性与安全性的影响。方法 计算机系统检索PubMed、SpringerLink、The Cochrane Library、OVID、中国知网(CNKI)、万方数据知识服务平台、中国生物医学文献数据库(SinoMed)、维普中文科技期刊数据库(VIP)和手动检索关于GEM和EPI治疗NMIBC的随机对照试验(RCT)。语言限定为中文和英文文章,检索时限为建库至2021年9月1日。采用RevMan5.3软件对GEM组与EPI组患者的术后肿瘤复发率、膀胱刺激征、肝肾功能损害,血尿和其他并发症进行meta分析,使用敏感性分析评价研究结果稳定性,发表偏倚使用漏斗图进行分析。结果 共纳入12篇RCT,包括1383例患者。Meta分析结果显示:GEM组患者术后肿瘤复发率[RR=0.78,95%CI(0.63,0.95),P=0.01]、膀胱刺激征发生率[RR=0.53,95%CI(0.42,0.67),P< 0.000 01]、血尿发生率[RR=0.41,95%CI(0.25,0.66),P=0.000 3]、肝肾功能损害[RR=0.29,95%CI(0.10,0.83),P=0.02]、其他不良反应(胃肠道反应、过敏、发热及皮疹等)发生率[RR=0.34,95%CI(0.20,0.56),P< 0.000 1]均低于EPI组,差异均有统计学意义(P< 0.05)。结论 GEM在治疗效果和毒副作用均优于EPI,是临床上安全可靠的肿瘤化疗药物。因此,建议今后在临床对TURBT术后的NMIBC患者使用GEM行灌注化疗,改善其预后。Abstract: Objective To systematically evaluate the efficacy and safety of gemcitabine(GEM) and epirubicin(EPI) after infusion chemotherapy in patients with non-muscle invasive bladder cancer(NMIBC).Methods The randomized controlled trials(RCTs) of GEM and EPI in the treatment of NMIBC in PubMed, SpringerLink, the Cochrane Library, Ovid, CNKI, Wanfang Data knowledge service platform, SinoMed, VIP and manual retrieval were searched by computer system. The language is limited to Chinese and English articles, and the retrieval time limit is from the establishment of the database to September 1, 2021. Revman5.3 software was used to meta analyze the postoperative tumor recurrence rate, bladder irritation symptoms, liver and kidney function damage, hematuria and other complications in GEM group and EPI group. Sensitivity analysis was used to evaluate the stability of the research results, and publication bias was analyzed by funnel diagram.Results A total of 12 RCTs were included, including 1383 patients. The results of meta-analysis showed that the postoperative tumor recurrence rate[RR=0.78, 95%CI(0.63, 0.95),P=0.01], the incidence of bladder irritation sign[RR=0.53, 95%CI(0.42, 0.67),P< 0.000 01], the incidence of hematuria[RR=0.41, 95%CI(0.25, 0.66),P=0.000 3], liver and kidney function damage[RR=0.29, 95%CI(0.10, 0.83),P=0.02]and other adverse reactions (gastrointestinal reaction, allergy, fever and rash, etc)[RR=0.34, 95%CI(0.20, 0.56),P< 0.000 1]in GEM group were lower than that in EPI group, and the differences were statistically significant(P< 0.05).Conclusion GEM is a safe and reliable tumor chemotherapy drug with better therapeutic effect and toxic and side effects than EPI. Therefore, it is suggested that GEM should be used for perfusion chemotherapy in NMIBC patients after TURBT to improve their prognosis in the future.
-
Key words:
- bladder cancer /
- gemcitabine /
- epirubicin /
- intravesical instillation /
- systematic review
-

-
表 1 吉西他滨和表柔比星灌注治疗膀胱癌临床效果的meta分析的文献基本特征表
作者及发表年份 研究类型 样本量/例 分组/例 性别/例 年龄/岁 肿瘤生长情况/例 肿瘤WHO分级 TNM分期 结局指标 GEM EPI 男 女 单发 多发 G1 G2 G3 Tis Ta T1 朱金光2021 RCT 68 34 34 46 22 39~80 42 26 NA NA NA NA NA NA ①②③⑤ 王安莲2020 RCT 300 150 150 218 82 43~79 NA NA 72 131 97 20 110 168 ①②③④⑤ 陈铁定2020 RCT 105 53 52 74 31 36~73 63 42 NA NA NA NA NA NA ①②③④⑤ 付炯2020 RCT 60 30 30 NA NA NA NA NA NA NA NA NA NA NA ①③⑤ 杜科霖2018 RCT 45 25 20 29 16 40~91 NA NA NA NA NA NA NA NA ①②③④⑤ 刘彦军2018 RCT 78 39 39 71 7 55~80 NA NA NA NA NA NA NA NA ①② 王晴2018 RCT 208 104 104 163 45 20~79 106 109 48 78 82 NA 109 99 ①②⑤ 胡小燕2017 RCT 165 89 76 140 25 45~75 120 45 NA NA 28 5 NA 27 ①②③④⑤ 胡卫锋2017 RCT 80 40 40 68 12 45~82 57 23 25 53 2 3 12 65 ①②③⑤ 冯勇2017 RCT 128 65 63 68 60 21~78 93 35 21 102 5 3 26 99 ①②③④⑤ 关豪2017 RCT 88 31 57 NA NA 24~88 NA NA NA NA NA NA NA NA ①②③⑤ 张小红2015 RCT 58 27 31 46 12 35~74 40 18 13 41 4 1 11 46 ②③④⑤ 注:RCT:随机对照试验;NA:数据不可获取或不可用。①肿瘤复发率;②膀胱刺激征;③血尿;④肝功能损害;⑤其他不良反应(胃肠道反应、过敏、发热及皮疹等)。 -
[1] Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends[J]. Eur Urol, 2017, 71(1): 96-108. doi: 10.1016/j.eururo.2016.06.010
[2] Bhanvadia SK. Bladder Cancer Survivorship[J]. Curr Urol Rep, 2018, 19(12): 111. doi: 10.1007/s11934-018-0860-6
[3] 郭学敬, 王民, 师磊. 老年浅表性膀胱癌患者经尿道膀胱肿瘤电切术后吉西他滨与吡柔比星膀胱热灌注的临床观察[J]. 中华老年多器官疾病杂志, 2018, 17(10): 762-765. doi: 10.11915/j.issn.1671-5403.2018.10.175
[4] 梁宇, 沈群山, 张珺, 等. 二次电切联合吉西他滨即刻灌注治疗非肌层浸润性膀胱癌的临床效果[J]. 安徽医学, 2019, 40(6): 609-612. doi: 10.3969/j.issn.1000-0399.2019.06.003
[5] Lu J, Xue Y, Shen F, et al. Transurethral holmium laser resection and transurethral electrocision combined with intravesical epirubicin within 24 hours postoperatively for treatment of bladder cancer[J]. J Int Med Res, 2020, 48(6): 1219686819.
[6] 朱金光, 张凯忠. 非肌层浸润型膀胱癌TUR-BT术后吉西他滨膀胱热灌注化疗的效果观察[J]. 海南医学, 2021, 32(1): 61-64. doi: 10.3969/j.issn.1003-6350.2021.01.016
[7] 王安莲, 刘赟, 胡世松, 等. 表柔比星与吉西他滨膀胱灌注化疗对浅表性膀胱尿路上皮癌术后复发及安全性的影响[J]. 国际泌尿系统杂志, 2020, 40(3): 478-481. doi: 10.3760/cma.j.cn431460-20190710-00023
[8] 陈铁定, 陈力, 张鹏. 膀胱肿瘤电切术后应用吉西他滨行膀胱灌注化疗的效果观察及对肿瘤标志物和生命质量的影响[J]. 中国医师进修杂志, 2020, 43(4): 364-367. doi: 10.3760/cma.j.cn115455-20190918-00688
[9] 付炯, 贾锐, 杜一鸣, 等. 等离子电切剜除联合吉西他滨局部灌注治疗浅表膀胱癌的效果分析[J]. 局解手术学杂志, 2020, 29(6): 456-460. https://www.cnki.com.cn/Article/CJFDTOTAL-JJXZ202006007.htm
[10] 杜科霖, 刘钦文, 杜国防, 等. TUR-BT术后吉西他滨膀胱灌注治疗膀胱癌的临床疗效分析[J]. 中国保健营养, 2018, 28(3): 301.
[11] 刘彦军, 景治安, 毛长青, 等. 吉西他滨与表柔比星对中高危非浸润性膀胱尿路上皮癌的疗效观察[J]. 实用癌症杂志, 2018, 33(12): 2059-2061. doi: 10.3969/j.issn.1001-5930.2018.12.043
[12] 王晴. 吉西他滨与表柔比星膀胱灌注化疗的疗效对比[D]. 天津: 天津医科大学, 2018.
[13] 胡小燕, 陈艺, 应敏, 等. 高危非肌层浸润性膀胱癌术后表柔比星与吉西他滨膀胱灌注的疗效分析[J]. 华南国防医学杂志, 2017, 31(6): 383-386, 393. https://www.cnki.com.cn/Article/CJFDTOTAL-HNGY201706008.htm
[14] 胡卫锋, 郭永连, 陈琳, 等. 非肌层浸润性膀胱癌术后吉西他滨与表柔比星膀胱灌注化疗疗效及安全性分析[J]. 现代泌尿生殖肿瘤杂志, 2017, 9(5): 269-271. doi: 10.3870/j.issn.1674-4624.2017.05.004
[15] 冯勇, 张治国, 李伟, 等. 非肌层浸润性膀胱癌术后吉西他滨膀胱灌注化疗的疗效分析[J]. 现代泌尿外科杂志, 2017, 22(5): 336-338, 348. doi: 10.3969/j.issn.1009-8291.2017.05.006
[16] 关豪. 非肌层浸润性膀胱癌术后药物灌注化疗的疗效分析[D]. 沈阳: 中国医科大学, 2017.
[17] 张小红, 谭靖, 姚鲲, 等. 抗肿瘤药物灌注预防表浅性膀胱癌术后复发的临床效果研究[J]. 中国医师杂志, 2015, 17(7): 1043-1045. doi: 10.3760/cma.j.issn.1008-1372.2015.07.026
[18] Soukup V, apoun O, Cohen D, et al. Risk Stratification Tools and Prognostic Models in Non-muscle-invasive Bladder Cancer: A Critical Assessment from the European Association of Urology Non-muscle-invasive Bladder Cancer Guidelines Panel[J]. Eur Urol Focus, 2020, 6(3): 479-489. doi: 10.1016/j.euf.2018.11.005
[19] Ouzaid I, Panthier F, Hermieu J F, et al. Contemporary surgical and technical aspects of transurethral resection of bladder tumor[J]. Transl Androl Urol, 2019, 8(1): 21-24. doi: 10.21037/tau.2019.01.04
[20] Kaufman DS, Shipley WU, Feldman AS. Bladder cancer[J]. Lancet, 2009, 374(9685): 239-249. doi: 10.1016/S0140-6736(09)60491-8
[21] Marttila T, Järvinen R, Liukkonen T, et al. Intravesical Bacillus Calmette-Guérin Versus Combination of Epirubicin and Interferon-α2a in Reducing Recurrence of Non-Muscle-invasive Bladder Carcinoma: FinnBladder-6 Study[J]. Eur Urol, 2016, 70(2): 341-347. doi: 10.1016/j.eururo.2016.03.034
[22] Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)-2019 Update[J]. Eur Urol, 2019, 76(5): 639-657. doi: 10.1016/j.eururo.2019.08.016
[23] Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline[J]. J Urol, 2016, 196(4): 1021-1029. doi: 10.1016/j.juro.2016.06.049
[24] Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guérin[J]. Eur Urol, 2016, 69(1): 60-69. doi: 10.1016/j.eururo.2015.06.045
[25] 赵振华, 赵国平, 郑东升, 等. 丝裂霉素膀胱灌注热化疗治疗T1G3膀胱尿路上皮癌的疗效分析[J]. 临床泌尿外科杂志, 2020, 35(2): 99-102. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMW202002003.htm
[26] Rouprêt M, Pignot G, Masson-Lecomte A, et al. French ccAFU guidelines-update 2020-2022: bladder cancer[J]. Prog Urol, 2020, 30(12S): S78-S135.
[27] 秦晓平, 詹雄宇, 陈奇彪, 等. 小檗碱增强丝裂霉素C诱导的膀胱癌T24细胞周期阻滞及凋亡[J]. 中国病理生理杂志, 2018, 34(6): 1025-1030. doi: 10.3969/j.issn.1000-4718.2018.06.011
[28] 彭磊, 蒙春杨, 李金泽, 等. 吉西他滨较丝裂霉素治疗TURBT后非肌层浸润性膀胱癌的复发率低、毒副作用小: 基于随机对照试验的荟萃分析[J]. 中国全科医学, 2021, 24(23): 2978-2984. doi: 10.12114/j.issn.1007-9572.2021.01.201
[29] Di Lorenzo G, Perdonà S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial[J]. Cancer, 2010, 116(8): 1893-1900. doi: 10.1002/cncr.24914
[30] Messing EM, Tangen CM, Lerner SP, et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial[J]. JAMA, 2018, 319(18): 1880-1888. doi: 10.1001/jama.2018.4657
[31] Prasanna T, Craft P, Balasingam G, et al. Intravesical Gemcitabine versus Intravesical Bacillus Calmette-Guérin for the Treatment of Non-Muscle Invasive Bladder Cancer: An Evaluation of Efficacy and Toxicity[J]. Front Oncol, 2017, 7(11): 260-265.
[32] Tabayoyong WB, Kamat AM, O'Donnell MA, et al. Systematic Review on the Utilization of Maintenance Intravesical Chemotherapy in the Management of Non-muscle-invasive Bladder Cancer[J]. Eur Urol Focus, 2018, 4(4): 512-521. doi: 10.1016/j.euf.2018.08.019
[33] 吴海超, 陈振杰, 付什, 等. 长期与短期膀胱内灌注表柔比星预防非肌层浸润性膀胱癌复发的比较: Meta分析和系统评价[J]. 临床泌尿外科杂志, 2021, 36(10): 812-819. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMW202110012.htm
[34] 郑万祥, 高学林, 侯广东, 等. 膀胱低度恶性潜能乳头状尿路上皮肿瘤的复发进展因素分析[J]. 中华泌尿外科杂志, 2020, 41(1): 8-12. doi: 10.3760/cma.j.issn.1000-6702.2020.01.002
[35] 李前进, 拜合提亚·阿扎提, 高新, 等. 表柔比星与吉西他滨膀胱灌注预防非肌层浸润性膀胱癌电切术后复发和进展的效果对比分析[J]. 医药前沿, 2020, 10(29): 112-113. https://www.cnki.com.cn/Article/CJFDTOTAL-HNYX201912056.htm
[36] 白云金, 杨玉帛, 韩平, 等. 吉西他滨膀胱灌注治疗复发性非肌层浸润性膀胱癌的疗效分析[J]. 现代泌尿外科杂志, 2016, 21(1): 9-11. doi: 10.3969/j.issn.1009-8291.2016.01.003
[37] Addeo R, Caraglia M, Bellini S, et al. Randomized phase Ⅲ trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance[J]. J Clin Oncol, 2010, 28(4): 543-548. doi: 10.1200/JCO.2008.20.8199
[38] 刘东操, 石洪波, 陈斌. 吉西他滨与吡柔比星行膀胱灌注治疗膀胱癌的对照研究[J]. 实用癌症杂志, 2018, 33(2): 328-330. doi: 10.3969/j.issn.1001-5930.2018.02.046
[39] 刘光涛, 李菲菲, 黄贵闽, 等. 吉西他滨、丝裂霉素、吡柔比星膀胱灌注预防非肌层浸润性膀胱癌术后复发的疗效分析[J]. 心理医生, 2017, 23(29): 31-32.
-




 下载:
下载: