Growth inhibition and the mechanisms of castration-resistant prostate cancer 22Rv1 cells by ursolic acid
-
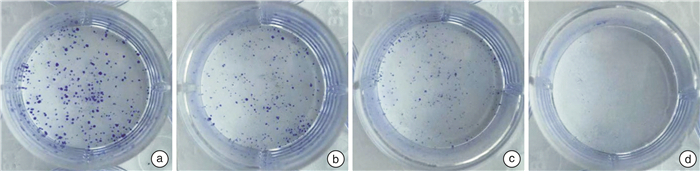
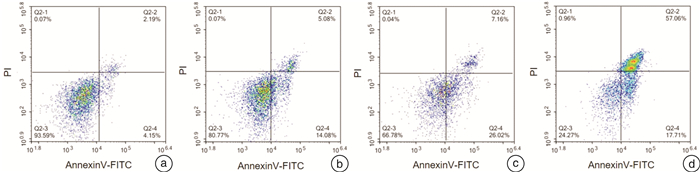
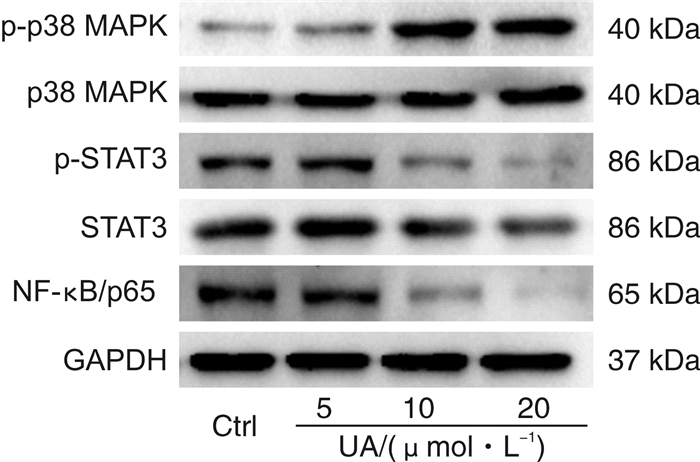
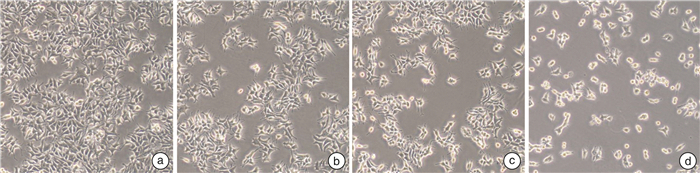
摘要: 目的 研究熊果酸(UA)对去势抵抗性前列腺癌22Rv1细胞生长的抑制作用并探讨其药理机制。方法 MTT法检测不同浓度UA分别作用22Rv1细胞12、24、48、72 h后对细胞的增殖抑制作用;倒置相差显微镜观察UA对22Rv1细胞形态的影响;克隆形成实验检测UA对22Rv1细胞平板克隆形成的影响;流式细胞术检测UA对22Rv1细胞凋亡的影响;Western印迹法检测UA对22Rv1细胞p38 MAPK蛋白和磷酸化、STAT3蛋白和磷酸化,以及NF-κB/p65蛋白表达的影响。结果 UA能显著抑制22Rv1细胞增殖,且抑制效应呈剂量和时间依赖性(P < 0.05);以5、10、20 μmol/L作为UA低、中、高浓度干预24 h,显微镜观察发现随着UA浓度增大,22Rv1细胞数目显著减少,细胞皱缩、质膜起泡;克隆形成实验结果显示,UA能显著减少22Rv1细胞克隆群落形成(P < 0.05);流式细胞术结果显示,UA能显著诱导22Rv1细胞凋亡细胞比例增多(P < 0.05);Western印迹结果显示,UA能增强p38 MAPK的磷酸化,并且抑制STAT3的磷酸化和NF-κB/p65蛋白的表达。结论 UA可抑制去势抵抗性前列腺癌22Rv1细胞的生长,促进22Rv1细胞凋亡,p38 MAPK/STAT3/NF-κB信号通路参与调控UA对22Rv1细胞的生长抑制作用。Abstract: Objective To study the growth inhibition effect of ursolic acid (UA) in the castration-resistant prostate cancer (CRPC) 22Rv1 cells and to explore its pharmacological mechanisms.Methods MTT assay was conducted to detect the proliferation, inverted phase contrast microscope was used to observe the morphology, colony formation assay was performed to examine the colony formation, flow cytometry was carried out to measure the apoptosis, and Western blotting was conducted to examine proteins expression of p-and p38 MAPK, p-and STAT3, as well as NF-κB/p65.Results UA significantly inhibited the proliferation of 22Rv1 cells, and the inhibitory effect displayed a dose-and time-dependent manner (P < 0.05). Regarding the doses of 5, 10, and 20 μmol/L as low, medium, and high concentrations of UA for the treatment of 24 h, the microscopic morphology showed significantly reduced number of 22Rv1 cells with shrunken cells and plasma membrane blebs upon treatment of increasing concentrations of UA; colony formation assay revealed that UA significantly reduced the colony formation of 22Rv1 cells (P < 0.05); flow cytometry analysis showed that UA significantly increased the proportion of apoptotic cells in 22Rv1 cells (P < 0.05); Western blotting analysis demonstrated that UA enhanced the phosphorylation of p38 MAPK, and supressed the phosphorylation of STAT3 and NF-κB/p65 protein expression.Conclusion UA exerts growth inhibition effect and promotes the apoptosis in CRPC 22Rv1 cells, and the p38 MAPK/STAT3/NF-κB signaling pathway is involved in regulating the growth inhibition effect of UA in 22Rv1 cells.
-

-
表 1 递增浓度UA处理细胞不同时间对22Rv1细胞增殖的影响
%,x±s 时间 细胞增殖 对照组(0 μmol/L) 5 μmol/L UA 10 μmol/L UA 15 μmol/L UA 20 μmol/L UA 30 μmol/L UA 12 h 100.00±13.55 120.09±5.841) 103.51±5.55 95.04±2.861) 73.02±3.511) 51.50±6.661) 24 h 100.00±3.23 94.90±4.25 71.85±5.971) 57.51±2.581) 44.27±2.291) 25.00±1.811) 48 h 100.00±6.12 96.77±8.04 73.90±2.941) 18.01±1.081) 10.59±0.561) 8.66±0.471) 72 h 100.00±4.43 85.91±2.861) 35.98±4.271) 7.43±0.331) 6.07±0.181) 6.28±0.621) 在相同时间点不同浓度下与对照组(0 μmol/L)比较,1)P<0.05。 表 2 不同浓度UA对22Rv1细胞克隆形成及凋亡的影响
x±s 项目 对照组(0 μmol/L) 5 μmol/L UA 10 μmol/L UA 20 μmol/L UA 克隆数目 457.67±25.48 328.00±28.581) 142.67±31.391) 9.00±6.001) 凋亡率/% 7.49±1.00 21.59±2.171) 27.09±6.001) 70.10±5.441) 与对照组(0 μmol/L)比较,1)P<0.05。 -
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. doi: 10.3322/caac.21492
[2] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. doi: 10.3322/caac.21338
[3] Karantanos T, Evans CP, Tombal B, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level[J]. Eur Urol, 2015, 67(3): 470-479. doi: 10.1016/j.eururo.2014.09.049
[4] 贾泽鹏, 陈泽宇, 高旭. 去势抵抗性前列腺癌的治疗进展[J]. 临床泌尿外科杂志, 2020, 35(4): 312-316, 320. https://lcmw.chinajournal.net.cn/WKC/WebPublication/paperDigest.aspx?paperID=f48e31c5-189a-4856-bca9-80fb6affdd37
[5] Shanmugam MK, Dai X, Kumar AP, et al. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies[J]. Biochem Pharmacol, 2013, 85(11): 1579-1587. doi: 10.1016/j.bcp.2013.03.006
[6] Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development[J]. Nat Rev Cancer, 2009, 9(8): 537-549. doi: 10.1038/nrc2694
[7] Eluard B, Thieblemont C, Baud V. NF-κB in the New Era of Cancer Therapy[J]. Trends Cancer, 2020, 6(8): 677-687. doi: 10.1016/j.trecan.2020.04.003
[8] Mohassab AM, Hassan HA, Abdelhamid D, et al. STAT3 transcription factor as target for anti-cancer therapy[J]. Pharmacol Rep, 2020, 72(5): 1101-1124. doi: 10.1007/s43440-020-00156-5
[9] Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy[J]. N Engl J Med, 2012, 367(13): 1187-1197. doi: 10.1056/NEJMoa1207506
[10] de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer[J]. N Engl J Med, 2011, 364(21): 1995-2005. doi: 10.1056/NEJMoa1014618
[11] Zhou J, Wang Y, Xiang S, et al. Towards understanding androgen receptor-independent prostate cancer: an evolving paradigm[J]. Transl Cancer Res, 2020, 9(2): 415-417. doi: 10.21037/tcr.2020.01.25
[12] Kim K, Shin EA, Jung JH, et al. Ursolic acid induces apoptosis in colorectal cancer cells partially via upregulation of MicroRNA-4500 and inhibition of JAK2/STAT3 phosphorylation[J]. Int J Mol Sci, 2018, 20(1): 1-13. doi: 10.3390/ijms20010001
[13] Guo JL, Han T, Bao L, et al. Ursolic acid promotes the apoptosis of cervical cancer cells by regulating endoplasmic reticulum stress[J]. J Obstet Gynaecol Res, 2019, 45(4): 877-881. doi: 10.1111/jog.13919
[14] Lin JH, Chen SY, Lu CC, et al. Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in gemcitabine-resistant human pancreatic cancer cells[J]. Phytother Res, 2020, 34(8): 2053-2066. doi: 10.1002/ptr.6669
[15] Zang YQ, Feng YY, Luo YH, et al. Glycitein induces reactive oxygen species-dependent apoptosis and G0/G1 cell cycle arrest through the MAPK/STAT3/NF-κB pathway in human gastric cancer cells[J]. Drug Dev Res, 2019, 80(5): 573-584.
-




 下载:
下载: