Establishment of a clinical prediction model for PSA gray zone prostate cancer: A study on the SEER database
-
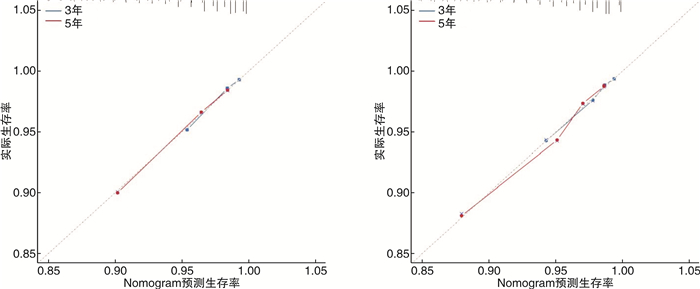
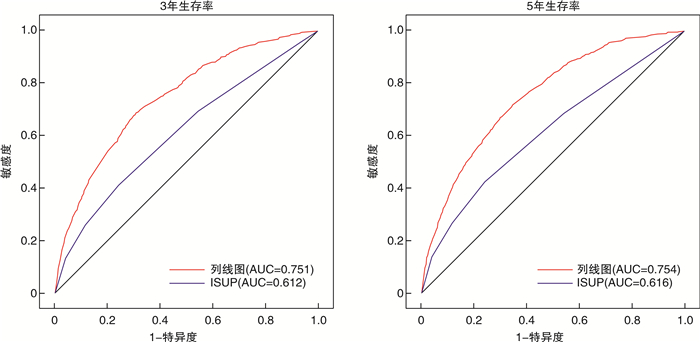
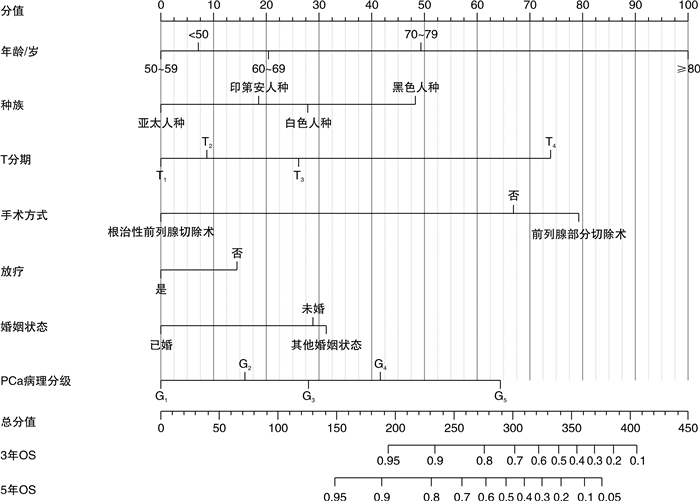
摘要: 目的 探讨前列腺特异抗原(PSA)灰区前列腺癌(PCa)患者预后的独立预测因素,并为其建立个体化预测的列线图模型。方法 回顾性检索2010—2016年PCa患者的临床资料,随机将其分为训练集(2/3)和验证集(1/3)。在多因素Cox回归分析的基础上,建立了独立危险因素的列线图。分别采用C指数(C-index)、ROC曲线及校准曲线来验证列线图预测准确性。结果 共收集PCa患者82 537例,其中训练集患者57 777例,验证集24 760例。多因素Cox分析显示,年龄、种族、婚姻状况、手术方式、是否放疗、是否化疗、病理分级及T分期是总生存期(OS)的独立危险因素。训练集OS的C指数(C-index)为0.741(95%CI:0.731~0.751),验证集为0.743(95%CI:0.725~0.761)。表明预测OS的列线图在训练集和验证集上都显示出较好的识别力。此外,ROC曲线及校准曲线验证了预测的生存概率与实际生存概率之间良好的一致性,并且其区分度优于传统病理分级系统。结论 本研究基于SEER数据库建立了国内外首个可以个体化预测PSA灰区PCa患者预后的列线图模型;且经内部及外部验证,其预测性能良好。列线图模型的建立将有助于辅助临床医生更加精准地预测PCa患者预后结局,为PCa患者个体化管理提供依据。Abstract: Objective To explore the independent predictors of the prognosis of prostate-specific antigen (PSA) gray zone prostate cancer (PCa) patients, and to establish a nomogram model for individualized prediction.Methods The clinical data of PCa patients from 2010 to 2016 were retrospectively searched, and they were randomly divided into a training set (2/3) and a validation set (1/3). On the basis of multivariate Cox regression analysis, a nomogram of independent risk factors was established. The C-index, ROC curve, and calibration curve were used to verify the accuracy of the nomogram prediction.Results A total of 82 537 PCa patients were collected, including 57 777 patients in the training set and 24 760 patients in the validation set. Multivariate Cox analysis showed that age, race, marital status, surgical method, radiotherapy, chemotherapy, pathological grade, and T stage were independent risk factors for overall survival (OS). The C-index of the training set OS was 0.741 (95%CI: 0.731-0.751), and the validation set was 0.743 (95%CI: 0.725-0.761). The nomogram of predicting OS showed good recognition ability on both the training set and the validation set. In addition, the ROC curve and calibration curve verified the good agreement between the predicted survival probability and the actual survival probability, and its discrimination was better than traditional pathological grading systems.Conclusion Based on the SEER database, this study established the first domestic and foreign nomogram model that could individually predict the prognosis of PCa patients in the PSA gray zone. Through internal and external verification, its prediction performance is good. The establishment of the nomogram model will help clinicians to more accurately predict the prognosis of PCa patients and provide a basis for individualized management of PCa patients.
-
Key words:
- prostate cancer /
- prostate specific antigen /
- nomogram /
- prognosis /
- SEER database
-

-
表 1 82 537例患者人口统计和临床特征
例(%) 变量 训练集(n=57 777) 验证集(n=24 760) P 年龄/岁 0.96 < 50 1521(2.6) 636(2.6) 50~59 14 160(24.5) 6028(24.3) 60~69 27 918(48.3) 12 006(48.5) 70~79 12 667(21.9) 5435(22.0) ≥80 1511(2.6) 655(2.6) 种族 0.136 白色人种 46 450(80.4) 19 852(80.2) 黑色人种 7376(12.8) 3281(13.3) 印第安人种 270(0.5) 103(0.4) 亚太人种 3681(6.4) 1524(6.2) 病理分级 0.327 Ⅰ 25 774(44.6) 10 962(44.3) Ⅱ 17 426(30.2) 7584(30.6) Ⅲ 7356(12.7) 3103(12.5) Ⅳ 4596(8.0) 1931(7.8) Ⅴ 2625(4.5) 1180(4.8) 临床分期 0.508 Ⅰ 17 952(31.1) 7614(30.8) Ⅱ 33 031(57.2) 14 157(57.2) Ⅲ 5913(10.2) 2589(10.5) Ⅳ 881(1.5) 400(1.6) T分期 0.317 T1 25 027(43.3) 10 582(42.7) T2 26 235(45.4) 11 302(45.6) T3 6388(11.1) 2824(11.4) T4 127(0.2) 52(0.2) N分期 0.208 N0 57 008(98.7) 24 403(98.6) N1 769(1.3) 357(1.4) 手术方式 0.644 未手术 31 827(55.1) 13 554(54.7) 前列腺部分切除术 1602(2.8) 685(2.8) 根治性前列腺切除术 24 348(42.1) 10 521(42.5) 放疗 0.421 是 20 393(35.3) 8667(35.0) 否 37 384(64.7) 16 093(65.0) 化疗 0.193 是 92(0.2) 38(0.1) 否 57 685(99.8) 24 722(99.9) 婚姻状态 0.11 已婚 44 103(76.3) 19 067(77.0) 未婚 6804(11.8) 2826(11.4) 其他婚姻状态 6870(11.9) 2867(11.6) 表 2 PSA灰区PCa患者预后的Cox单因素及多因素回归分析
变量 单因素分析(n=57 777) 多因素分析(n=57 777) HR(95%CI) P HR(95%CI) P 年龄/岁 < 50 Ref Ref Ref Ref 50~59 0.891(0.613~1.295) 0.544 0.888(0.610~1.291) 0.533 60~69 1.447(1.010~2.072) < 0.05 1.280(0.892~1.837) 0.180 70~79 3.070(2.143~4.399) < 0.001 2.152(1.494~3.101) < 0.001 ≥80 11.212(7.738~16.245) < 0.001 5.402(3.687~7.915) < 0.001 种族 白色人种 Ref Ref Ref Ref 黑色人种 1.434(1.276~1.612) < 0.001 1.441(1.277~1.627) < 0.001 印第安人种 0.984(0.511~1.900) 0.962 0.837(0.434~1.614) 0.596 亚太人种 0.651(0.519~0.817) < 0.001 0.611(0.486~0.768) < 0.001 病理分级 Ⅰ Ref Ref Ref Ref Ⅱ 1.283(1.146~1.436) < 0.001 1.279(1.036~1.579) < 0.05 Ⅲ 1.781(1.555~2.039) < 0.001 1.578(1.258~1.978) < 0.001 Ⅳ 2.482(2.152~2.863) < 0.001 1.578(1.570~2.501) < 0.001 Ⅴ 4.377(3.780~5.068) < 0.001 2.890(2.268~3.683) < 0.001 临床分期 Ⅰ Ref Ref Ref Ref Ⅱ 1.157(1.048~1.278) < 0.01 1.051(0.842~1.313) 0.659 Ⅲ 0.833(0.699~0.992) < 0.01 0.859(0.463~1.593) 0.628 Ⅳ 3.124(2.448~3.986) < 0.001 1.177(0.515~2.691) 0.699 T分期 T1 Ref Ref Ref Ref T2 0.629(0.574~0.690) < 0.001 1.166(1.046~1.298) < 0.01 T3 0.664(0.569~0.775) < 0.001 1.826(1.026~3.249) < 0.05 T4 4.766(3.124~7.270) < 0.001 2.967(1.414~6.229) < 0.01 N分期 N0 Ref Ref Ref Ref N1 2.557(1.960~3.335) < 0.001 1.583(0.756~3.314) 0.223 手术方式 未手术 Ref Ref Ref Ref 前列腺部分切除术 1.954(1.645~2.321) < 0.001 1.247(1.039~1.497) < 0.05 根治性前列腺切除术 0.317(0.285~0.353) < 0.001 0.297(0.251~0.351) < 0.001 放疗 是 Ref Ref Ref Ref 否 0.588(0.539~0.642) < 0.001 1.295(1.164~1.440) < 0.001 化疗 是 Ref Ref Ref Ref 否 0.374(0.178~0.785) < 0.01 0.624(0.295~1.318) 0.216 婚姻状态 已婚 Ref Ref Ref Ref 未婚 1.637(1.443~1.856) < 0.001 1.674(1.472~1.90) < 0.001 其他婚姻状态 2.231(2.002~2.486) < 0.001 1.748(1.566~1.952) < 0.001 -
[1] Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022[J]. CA Cancer J Clin, 2022.
[2] Fitzmaurice C, Allen C, Barber R M, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study[J]. JAMA Oncol, 2017, 3(4): 524-548. doi: 10.1001/jamaoncol.2016.5688
[3] US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement[J]. JAMA, 2018, 319(18): 1901-1913. doi: 10.1001/jama.2018.3710
[4] Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force[J]. Ann Intern Med, 2011, 155(11): 762-771. doi: 10.7326/0003-4819-155-11-201112060-00375
[5] Fenton JJ, Weyrich MS, Durbin S, et al. Prostate-Specific Antigen-Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force[J]. JAMA, 2018, 319(18): 1914-1931. doi: 10.1001/jama.2018.3712
[6] Alonzo DG, Mure AL, Soloway MS. Prostate cancer and the increasing role of active surveillance[J]. Postgrad Med, 2013, 125(5): 109-116. doi: 10.3810/pgm.2013.09.2705
[7] Inahara M, Suzuki H, Kojima S, et al. Improved prostate cancer detection using systematic 14-core biopsy for large prostate glands with normal digital rectal examination findings[J]. Urology, 2006, 68(4): 815-819. doi: 10.1016/j.urology.2006.05.010
[8] Stattin P, Carlsson S, Holmström B, et al. Prostate cancer mortality in areas with high and low prostate cancer incidence[J]. J Natl Cancer Inst, 2014, 106(3): dju007. doi: 10.1093/jnci/dju007
[9] Cabarkapa S, Perera M, McGrath S, et al. Prostate cancer screening with prostate-specific antigen: A guide to the guidelines[J]. Prostate Int, 2016, 4(4): 125-129. doi: 10.1016/j.prnil.2016.09.002
[10] Crawford ED, Ventii K, Shore ND. New biomarkers in prostate cancer[J]. Oncology(Williston Park), 2014, 28(2): 135-142.
[11] Hou G, Zheng Y, Wei D, et al. Development and validation of a SEER-based prognostic nomogram for patients with bone metastatic prostate cancer[J]. Medicine(Baltimore), 2019, 98(39): e17197.
[12] Chen CH, Chang TT, Cheng KS, et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients[J]. Liver Int, 2006, 26(7): 766-773. doi: 10.1111/j.1478-3231.2006.01309.x
[13] Shah S, Boucai L. Effect of Age on Response to Therapy and Mortality in Patients With Thyroid Cancer at High Risk of Recurrence[J]. J Clin Endocrinol Metab, 2018, 103(2): 689-697. doi: 10.1210/jc.2017-02255
[14] Bernard B, Burnett C, Sweeney CJ, et al. Impact of age at diagnosis of de novo metastatic prostate cancer on survival[J]. Cancer, 2020, 126(5): 986-993. doi: 10.1002/cncr.32630
[15] Hoeijmakers JH. DNA damage, aging, and cancer[J]. N Engl J Med, 2009, 361(15): 1475-1485. doi: 10.1056/NEJMra0804615
[16] Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians[J]. Chin J Cancer, 2012, 31(9): 421-429. doi: 10.5732/cjc.011.10324
[17] Dess RT, Hartman HE, Mahal BA, et al. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality[J]. JAMA Oncol, 2019, 5(7): 975-983. doi: 10.1001/jamaoncol.2019.0826
[18] Petrovics G, Li H, Stümpel T, et al. A novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American men[J]. EBioMedicine, 2015, 2(12): 1957-1964. doi: 10.1016/j.ebiom.2015.10.028
[19] Bai DS, Chen P, Qian JJ, et al. Effect of marital status on the survival of patients with gallbladder cancer treated with surgical resection: a population-based study[J]. Oncotarget, 2017, 8(16): 26404-26413. doi: 10.18632/oncotarget.15476
[20] Zhou H, Zhang Y, Song Y, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: An analysis of the Surveillance, Epidemiology, and End Results(SEER)database[J]. Clin Res Hepatol Gastroenterol, 2017, 41(4): 476-486. doi: 10.1016/j.clinre.2017.02.008
[21] Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer[J]. J Clin Oncol, 2013, 31(31): 3869-3876. doi: 10.1200/JCO.2013.49.6489
[22] Zhou R, Yan S, Li J. Influence of marital status on the survival of patients with gastric cancer[J]. J Gastroenterol Hepatol, 2016, 31(4): 768-775. doi: 10.1111/jgh.13217
[23] Rendall MS, Weden MM, Favreault MM, et al. The protective effect of marriage for survival: a review and update[J]. Demography, 2011, 48(2): 481-506. doi: 10.1007/s13524-011-0032-5
[24] Manzoli L, Villari P, M Pirone G, et al. Marital status and mortality in the elderly: a systematic review and meta-analysis[J]. Soc Sci Med, 2007, 64(1): 77-94. doi: 10.1016/j.socscimed.2006.08.031
[25] 王义昆, 王林, 吴磊, 等. 前列腺癌放疗后复发患者行腹腔镜术的疗效及安全性分析[J]. 临床泌尿外科杂志, 2021, 36(9): 742-745, 748. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMW202109015.htm
[26] Podder TK, Fredman ET, Ellis RJ. Advances in Radiotherapy for Prostate Cancer Treatment[J]. Adv Exp Med Biol, 2018, 1096: 31-47.
[27] Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score[J]. Eur Urol, 2016, 69(3): 428-435. doi: 10.1016/j.eururo.2015.06.046
[28] Nazim SM, Abbas F. Role of Surgery in locally advanced prostate cancer[J]. Pak J Med Sci, 2015, 31(3): 710-716.
[29] Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent[J]. Eur Urol, 2017, 71(4): 618-629. doi: 10.1016/j.eururo.2016.08.003
[30] Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part Ⅱ: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer[J]. Eur Urol, 2017, 71(4): 630-642. doi: 10.1016/j.eururo.2016.08.002
[31] Brookman-May S, May M, Wieland WF, et al. Should we abstain from Gleason score 2-4 in the diagnosis of prostate cancer? Results of a German multicentre study[J]. World J Urol, 2012, 30(1): 97-103. doi: 10.1007/s00345-010-0632-5
[32] Montironi R, Cheng L, Lopez-Beltran A, et al. Original Gleason system versus 2005 ISUP modified Gleason system: the importance of indicating which system is used in the patient's pathology and clinical reports[J]. Eur Urol, 2010, 58(3): 369-373. doi: 10.1016/j.eururo.2010.04.028
[33] Epstein JI, Amin MB, Reuter VE, et al. Contemporary Gleason Grading of Prostatic Carcinoma: An Update With Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology(ISUP)Consensus Conference on Gleason Grading of Prostatic Carcinoma[J]. Am J Surg Pathol, 2017, 41(4): e1-e7. doi: 10.1097/PAS.0000000000000820
[34] Pierorazio PM, Walsh PC, Partin AW, et al. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system[J]. BJU Int, 2013, 111(5): 753-760. doi: 10.1111/j.1464-410X.2012.11611.x
[35] Wissing M, Brimo F, Chevalier S, et al. Optimization of the 2014 Gleason grade grouping in a Canadian cohort of patients with localized prostate cancer[J]. BJU Int, 2019, 123(4): 624-631. doi: 10.1111/bju.14512
[36] Offermann A, Hohensteiner S, Kuempers C, et al. Prognostic Value of the New Prostate Cancer International Society of Urological Pathology Grade Groups[J]. Front Med(Lausanne), 2017, 4: 157.
[37] Grogan J, Gupta R, Mahon KL, et al. Predictive value of the 2014 International Society of Urological Pathology grading system for prostate cancer in patients undergoing radical prostatectomy with long-term follow-up[J]. BJU Int, 2017, 120(5): 651-658. doi: 10.1111/bju.13857
[38] Wu X, Lv D, Lei M, et al. A 10-gene signature as a predictor of biochemical recurrence after radical prostatectomy in patients with prostate cancer and a Gleason score ≥7[J]. Oncol Lett, 2020, 20(3): 2906-2918. doi: 10.3892/ol.2020.11830
[39] Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye[J]. Lancet Oncol, 2015, 16(4): e173-e180. doi: 10.1016/S1470-2045(14)71116-7
[40] Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis[J]. J Clin Oncol, 2008, 26(8): 1364-1370. doi: 10.1200/JCO.2007.12.9791
[41] Roberto M, Botticelli A, Strigari L, et al. Prognosis of elderly gastric cancer patients after surgery: a nomogram to predict survival[J]. Med Oncol, 2018, 35(7): 111.
[42] Fang C, Wang W, Feng X, et al. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms[J]. Br J Cancer, 2017, 117(10): 1544-1550.
[43] Cao J, Yuan P, Wang L, et al. Clinical Nomogram for Predicting Survival of Esophageal Cancer Patients after Esophagectomy[J]. Sci Rep, 2016, 6: 26684.
[44] Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ[J]. J Clin Oncol, 2010, 28(23): 3762-3769.
-




 下载:
下载: