Establishment of IMDC-Olig model based on overall mortality to optimize candidates selection for cytoreductive nephrectomy
-
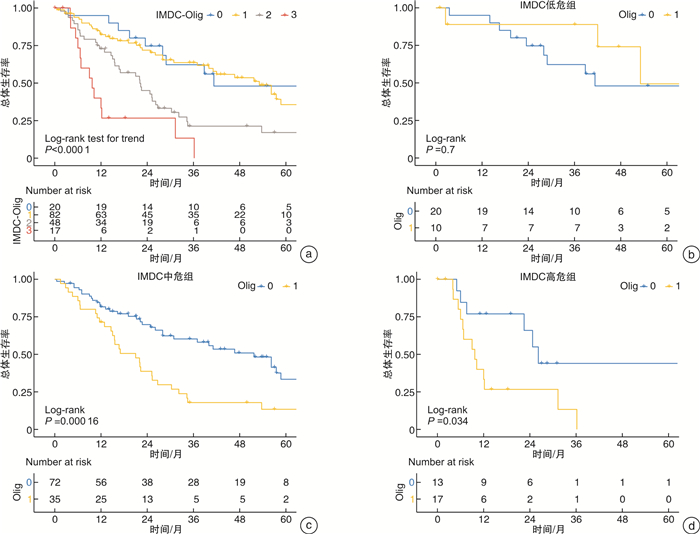
摘要: 目的 探索国际转移肾癌数据库联盟(International mRCC Database Consortium,IMDC)危险分层以外可以独立预测减瘤性肾切除术后患者总体死亡风险的因素,并构建联合预测模型,以指导手术适应证选择。方法 回顾性分析来自国际减瘤性肾切除术临床研究协作组(International Consortium of Clinical Research on Cytoreductive Nephrectomy)全球13个医学中心减瘤性肾切除术治疗转移性肾癌264例患者资料,中位年龄63岁,男性占比73.9%,66.7%的患者合并肺转移,26.5%的患者合并骨转移,寡转移患者占比73.3%,IMDC中高危占比63.3%。基于Cox多因素分析结果构建减瘤性肾切除术后患者总体死亡风险的临床预测模型,并计算IMDC模型和联合模型的C-index值。基于纳入模型的变量系数间比例关系进行半定量赋值,绘制新模型不同分值患者队列的生存曲线并进行log-rank检验,分析新纳入变量在IMDC危险分层不同亚组的预测价值。计算新模型预测3年死亡风险的时依性ROC曲线下面积。结果 开放手术占比68.9%,术后病理学肿瘤最大径中位9(7.1,12.3) cm,透明细胞癌占81.8%,手术切缘阳性率6.1%,清扫淋巴结阳性率47.7%,二期手术行转移灶切除比例为8.3%。中位随访27.5(15.1,46.4)个月,随访期内死亡110例,总体死亡率41.7%。Cox多因素回归结果显示,IMDC危险分层和寡转移状态(Oligo-metastasis,影像学评估转移病灶数不超过5个,HR=2.089,96%CI:1.390~3.139,P < 0.001)是患者术后总体死亡风险的独立预测因素。IMDC模型C-index值为0.598,IMDC-Olig联合模型C-index值为0.645。IMDC不同危险分层的亚组分析显示,在IMDC中危组和高危组,是否为寡转移两组患者术后总体死亡风险差异均有统计学意义(P < 0.05,高危组差异仅限于1年生存率),IMDC低危组两者差异无统计学意义(P>0.05)。结论 IMDC-Olig模型可以很好地对IMDC中高危组患者基于总体死亡风险进行再分层,可作为手术适应证选择的重要参考。Abstract: Objective To explore factors other than IMDC (International mRCC Database Consortium) risk stratification that can independently predict the overall mortality risk in patients following cytoreductive nephrectomy, and to construct a combined prediction model to guide the indication selection.Methods Patient's data from the International Consortium of Clinical Research on Cytoreductive Nephrectomy among 13 medical centers around the world with cytoreductive nephrectomy for the treatment of metastatic renal cancer were retrospectively analysed including 264 cases, with a median age of 63 years, male accounted for 73.9%. Lung metastases were found in 66.7% of patients, 26.5% had bone metastases, 73.3% of patients with oligo-metastases, and 63.3% of IMDC high-risk patients. Based on the results of Cox multivariate analysis, a clinical prediction model for the overall mortality risk of patients after cytoreductive nephrectomy was constructed, and the C-index value of the IMDC model and the combined model was calculated. Based on the proportional relationship between the coefficients of the variables included in the model, semi-quantitative assignment was performed, the survival curves of the cohort of patients with different scores in the new model were drawn, and the log-rank test was performed to analyze the predictive value of the newly included variables in different subgroups of IMDC risk stratification. The area under the time-dependent ROC curve of the new model for predicting 3-year mortality risk was calculated.Results Open surgery accounted for 68.9%, and the median maximum diameter of primary tumor on pathological examination was 9(7.1, 12.3) cm. Clear cell RCC accounted for 81.8%, the positive rate of surgical margins was 6.1%, and the positive rate of dissected lymph nodes was 47.7%. The rate of resection of metastases at secondary stage fashion was 8.3%. The median follow-up period was 27.5 (15.1, 46.4) months. One hundred and ten patients died during the follow-up period, and the overall mortality was 41.7%. Cox multivariate regression results showed that IMDC risk stratification and oligometastatic status (defined as the number of metastatic lesions assessed by imaging did not exceed 5, HR=2.089, 96%CI: 1.390-3.139, P < 0.001) were the independent predictors of overall mortality risk. The C-index value of the IMDC model was 0.598, and the C-index value of the IMDC-Olig joint model was 0.645. The subgroup analysis based on IMDC risk stratification showed that there was a statistically significant difference in the overall mortality risk regarding oligometastatic status in the intermediate and high-risk groups of IMDC(P < 0.05, but the difference in the high-risk group was limited to 1 year survival), while there was no significant difference regarding oligometastatic status in the IMDC low-risk group(P > 0.05).Conclusion The IMDC-Olig model can well re-stratify patients in the intermediate and high IMDC risk groups based on the overall risk of death, and can be used as an important reference for the selection of surgical indications.
-
Key words:
- cytoreductive nephrectomy /
- survival analysis /
- risk stratification /
- surgical indication
-

-
表 1 患者基线资料
M(Q1,Q3),例(%) 观察指标 数值 初诊年龄/岁 63(56,70) 男性 195(73.9) ECOG PS≥2 32(12.1) KPS < 80% 53(20.1) IMDC危险分层 低危 31(11.7) 中危 129(48.9) 高危 38(14.4) 未知 66(25.0) 临床T分期 cT1~2 101(38.2) cT3~4 161(61.0) cTx 2(0.8) 临床N分期 cN0 147(55.7) cN+ 112(42.4) cNx 5(1.9) 初诊时>5处转移病灶患者 71(26.9) 肺转移 176(66.7) 骨转移 70(26.5) 脑转移 7(2.7) 肝转移 21(8.0) 胰腺转移 10(3.8) 全身多器官转移 83(31.4) 术前接受系统治疗患者 22(8.3) 免疫学检查点抑制剂 6(2.3) TKI药物 16(6.1) 表 2 围术期资料和随访结果
M(Q1,Q3),例(%) 观察指标 数值 手术方式 肾切除术 243(92.0) 肾部分切除术 5(1.9) 肾切除术联合转移灶切除 16(6.1) 开放手术 182(68.9) 扩大淋巴结清扫(肾门范围以外) 68(25.8) 静脉癌栓切除 66(25.0) 周围脏器联合切除 59(22.3) 术中出血量/mL 150(100,500) 术中并发症 41(15.5) 术后并发症 78(29.5) 1~2级 68(25.8) 3~4级 9(3.4) 5级 1(0.4) 出院30 d再次入院 16(6.1) 术后影像学复查无可见病灶 19(7.2) 病理学肿瘤最大径/cm 9(7.1,12.3) 组织学类型 肾透明细胞癌 216(81.8) 肾乳头状细胞癌 20(7.6) 肾嫌色细胞癌 4(1.5) 其他恶性病变 24(9.1) 合并肉瘤样分化 49(18.6) 切缘阳性 16(6.1) 病理T分期 pT1~2 52(19.7) pT3~4 194(73.5) pTx 18(6.8) 病理N分期 pN0 100(37.9) pN+ 126(47.7) pNx 38(14.4) 二期手术行转移灶切除 22(8.3) 手术至术后接受系统治疗间隔/月 1.3(1,2.8) 术后免疫学检查点抑制剂治疗 一线治疗 27(10.2) 二线治疗 10(3.8) 三线治疗 14(5.3) 四线治疗 2(0.8) 术后TKI/mTOR药物治疗 一线治疗 123(46.6) 二线治疗 91(34.5) 三线治疗 53(20.1) 四线治疗 27(10.2) 术后免疫联合靶向治疗 一线治疗 5(1.9) 二线治疗 1(0.4) 三线治疗 0(0) 总体随访时间/月 27.5(15.1,46.4) 随访期内死亡 110(41.7) 表 3 Cox比例风险回归模型预测患者术后总体死亡风险
观察指标 单因素分析 多因素分析 HR(95%CI) P HR(95%CI) P 初诊年龄 0.994(0.976~1.013) 0.541 - 男性vs女性 1.081(0.700~1.667) 0.726 - ECOG PS < 2 vs ≥2 0.783(0.362~1.692) 0.534 KPS < 80% vs ≥80% 1.329(0.852~2.072) 0.209 - IMDC危险分层 低危 参照 参照 中危 2.239(1.204~4.161) 0.011 2.101(1.103~4.003) 0.024 高危 4.241(2.039~8.818) < 0.001 3.292(1.521~7.125) 0.002 初诊时转移病灶个数>5 vs ≤5 2.241(1.511~3.321) < 0.001 2.089(1.390~3.139) < 0.001 肺转移 1.349(0.867~2.097) 0.184 - 骨转移 0.967(0.633~1.477) 0.878 - 脑转移 0.963(0.237~3.913) 0.958 - 肝转移 1.144(0.575~2.274) 0.702 - 胰腺转移 0.424(0.131~1.364) 0.150 - 全身多器官转移 1.385(0.883~2.171) 0.156 - 术前接受系统治疗 0.757(0.276~2.080) 0.589 - 原发灶手术(微创vs开放) 0.884(0.546~1.432) 0.616 - 淋巴结清扫 不清扫 参照 - 肾门范围 1.230(0.735~2.059) 0.430 - 扩大清扫(至肾门范围以外) 1.063(0.643~1.757) 0.813 - 术中并发症 1.045(0.632~1.727) 0.864 - 术后并发症(2~5级) 0.265(0.834~1.937) 1.271 原发病灶病例类型(非透明vs透明) 0.940(0.456~1.938) 0.867 - 肉瘤样分化 1.312(0.816~2.111) 0.262 - 切缘阳性 1.866(0.969~3.595) 0.062 - 病理T分期 pT1~2 参照 参照 pT3~4 1.727(1.021~2.923) 0.047 1.432(0.819~2.502) 0.207 病理N分期 pN0 参照 - pN+ 1.319(0.889~1.957) 0.168 - 二期手术行转移灶切除 0.410(0.205~0.822) 0.012 0.562(0.265~1.187) 0.131 表 4 基于IMDC-Olig模型危险评分的患者生存率
疾病状态 IMDC-Olig
模型评分IMDC-Olig
危险分层1年总生存率
(95%CI)3年总生存率
(95%CI)5年总生存率
(95%CI)7年总生存率
(95%CI)IMDC低危+寡转移 0 低危 0.95
(0.86~1)0.62
(0.43~0.89)0.48
(0.29~0.8)0.48
(0.29~0.8)IMDC低危+非寡转移
IMDC中危+寡转移1 低危 0.82
(0.74~0.91)0.64
(0.53~0.76)0.36
(0.24~0.54)0.27
(0.15~0.47)IMDC中危+非寡转移
IMDC高危+寡转移2 中危 0.73
(0.61~0.87)0.21
(0.12~0.39)0.17
(0.08~0.36)0.11
(0.04~0.34)IMDC高危+非寡转移 3 高危 0.33
(0.16~0.68)0.13
(0.03~0.67)0(-) 0(-) -
[1] Motzer RJ, Russo P. Cytoreductive Nephrectomy-Patient Selection Is Key[J]. N Engl J Med, 2018, 379(5): 481-482. doi: 10.1056/NEJMe1806331
[2] Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update[J]. Eur Urol, 2022, S0302-2838(22)01676-1.
[3] Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study[J]. J Clin Oncol, 2009, 27(34): 5794-5799. doi: 10.1200/JCO.2008.21.4809
[4] Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study[J]. Lancet Oncol, 2013, 14(2): 141-148. doi: 10.1016/S1470-2045(12)70559-4
[5] Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial[J]. Lancet Oncol, 2021, 22(12): 1732-1739. doi: 10.1016/S1470-2045(21)00528-3
[6] Siva S, Bressel M, Wood ST, et al. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma-The RAPPORT Trial[J]. Eur Urol, 2022, 81(4): 364-372. doi: 10.1016/j.eururo.2021.12.006
[7] Assel M, Sjoberg D, Elders A, et al. Guidelines for Reporting of Statistics for Clinical Research in Urology[J]. Eur Urol, 2019, 75(3): 358-367. doi: 10.1016/j.eururo.2018.12.014
[8] Capitanio U, Abdollah F, Matloob R, et al. Effect of number and location of distant metastases on renal cell carcinoma mortality in candidates for cytoreductive nephrectomy: implications for multimodal therapy[J]. Int J Urol, 2013, 20(6): 572-579. doi: 10.1111/iju.12004
[9] Culp SH, Tannir NM, Abel EJ, et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy?[J]. Cancer, 2010, 116(14): 3378-3388. doi: 10.1002/cncr.25046
[10] McIntosh AG, Umbreit EC, Holland LC, et al. Optimizing patient selection for cytoreductive nephrectomy based on outcomes in the contemporary era of systemic therapy[J]. Cancer, 2020, 126(17): 3950-3960. doi: 10.1002/cncr.32991
[11] Yoon HJ, Paeng JC, Kwak C, et al. Prognostic implication of extrarenal metabolic tumor burden in advanced renal cell carcinoma treated with targeted therapy after nephrectomy[J]. Ann Nucl Med, 2013, 27(8): 748-755. doi: 10.1007/s12149-013-0742-4
[12] de Bruijn RE, Nijkamp J, Noe A, et al. Baseline tumor volume in assessing prognosis of patients with intermediate-risk synchronous metastatic renal cell carcinoma[J]. Urol Oncol, 2016, 34(6): 258.e7-258.e13. doi: 10.1016/j.urolonc.2015.12.007
[13] Marchioni M, Kriegmair M, Heck M, et al. Development of a Novel Risk Score to Select the Optimal Candidate for Cytoreductive Nephrectomy Among Patients with Metastatic Renal Cell Carcinoma. Results from a Multi-institutional Registry(REMARCC)[J]. Eur Urol Oncol, 2021, 4(2): 256-263. doi: 10.1016/j.euo.2020.12.010
-




 下载:
下载: