版权声明:本文原文“Pain and health-related quality of life with olaparib versus physician’s choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations(PROfound):an open-label,randomised,phase 3 trial”,首次发表在Lancet Oncol[2022,23(3):393-405];本次二次发表已取得版权所有者Ⓡ 2022 Elsevier出版社的同意,对原文进行摘译,不涉及一稿多投及侵犯版权等问题。
THE LANCET Oncology:奥拉帕利较新型内分泌药物对伴有同源重组修复基因突变的转移性去势抵抗性前列腺癌患者的疼痛和健康相关生活质量影响的PROfound研究:一项开放标签、随机、3期研究
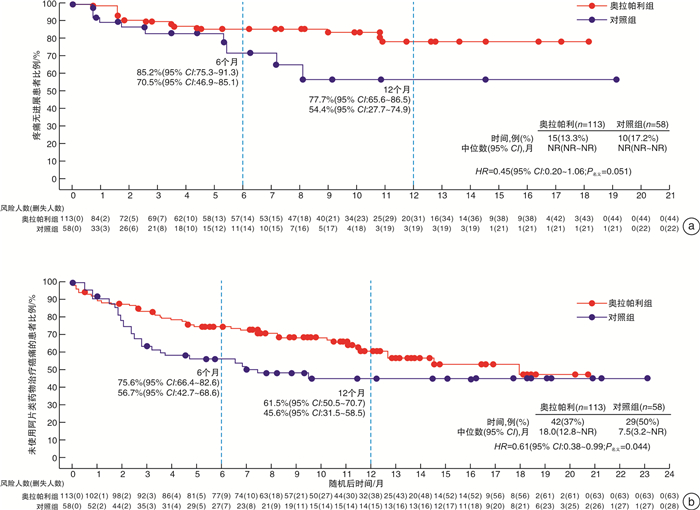
-
-

-
[1] Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer[J]. Cell, 2015, 161(5): 1215-1228. doi: 10.1016/j.cell.2015.05.001
[2] McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose)polymerase inhibition[J]. Cancer Res, 2006, 66(16): 8109-8115. doi: 10.1158/0008-5472.CAN-06-0140
[3] de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer[J]. N Engl J Med, 2020, 382(22): 2091-2102. doi: 10.1056/NEJMoa1911440
[4] Albala DM. Imaging and treatment recommendations in patients with castrate-resistant prostate cancer[J]. Rev Urol, 2017, 19(3): 200-202.
[5] Holmstrom S, Naidoo S, Turnbull J, et al. Symptoms and Impacts in Metastatic Castration-Resistant Prostate Cancer: Qualitative Findings from Patient and Physician Interviews[J]. Patient, 2019, 12(1): 57-67. doi: 10.1007/s40271-018-0349-x
[6] Burbridge C, Randall JA, Lawson J, et al. Understanding symptomatic experience, impact, and emotional response in recently diagnosed metastatic castration-resistant prostate cancer: a qualitative study[J]. Support Care Cancer, 2020, 28(7): 3093-3101. doi: 10.1007/s00520-019-05079-3
[7] Nussbaum N, George DJ, Abernethy AP, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science[J]. Prostate Cancer Prostatic Dis, 2016, 19(2): 111-121. doi: 10.1038/pcan.2015.42
[8] Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer[J]. N Engl J Med, 2020, 383(24): 2345-2357. doi: 10.1056/NEJMoa2022485
[9] Shore ND, Laliberté F, Ionescu-Ittu R, et al. Real-World Treatment Patterns and Overall Survival of Patients with Metastatic Castration-Resistant Prostate Cancer in the US Prior to PARP Inhibitors[J]. Adv Ther, 2021, 38(8): 4520-4540. doi: 10.1007/s12325-021-01823-6
[10] Thiery-Vuillemin A, de Bono J, Hussain M, et al. Pain and health-related quality of life with olaparib versus physician's choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations(PROfound): an open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2022, 23(3): 393-405. doi: 10.1016/S1470-2045(22)00017-1
[11] US Food & Drug Administration. Guidance for Industry: clinical trials endpoints for the approval of cancer drugs and biologics[EB/OL], (2007-05)[2021-08-31]. https://scimega.com/downloads/industry-reports/2007-05-Clinical-Trial-Endpoints-for-the-Approval-of-Cancer-Drugs-and-Biologics.pdf.
[12] European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man[EB/OL], (2014-06-17)[2021-08-31]. https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evaluation-anticancer-medicinal-products-man_en.pdf.
[13] Scher HI, Morris MJ, Stadler WM, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3[J]. J Clin Oncol, 2016, 34(12): 1402-1418. doi: 10.1200/JCO.2015.64.2702
[14] Beer TM, Miller K, Tombal B, et al. The association between health-related quality-of-life scores and clinical outcomes in metastatic castration-resistant prostate cancer patients: Exploratory analyses of AFFIRM and PREVAIL studies[J]. Eur J Cancer, 2017, 87: 21-29. doi: 10.1016/j.ejca.2017.09.035
-




 下载:
下载: