Evaluation of adjuvant chemotherapy for patients with ≥pT2 upper tract urothelial carcinoma after radical nephroureterectomy and prediction of postoperative intravesical recurrence
-
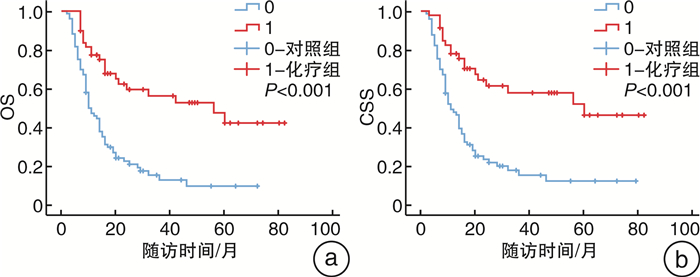
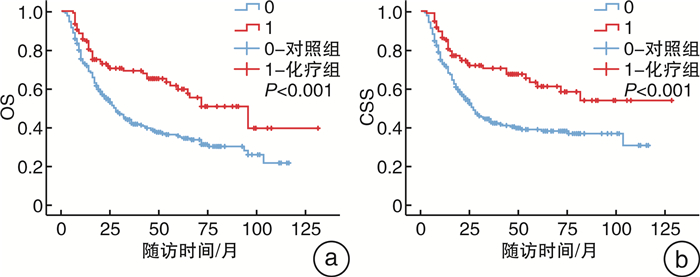
摘要: 目的 确定术后pT2~4NxM0期的上尿路尿路上皮癌(UTUC)患者是否受益于根治性肾输尿管切除术(RNU)后90 d内开始的辅助化疗,并寻找UTUC患者术后膀胱复发的危险因素。方法 回顾性分析2011年8月—2021年8月在徐州医科大学附属医院接受RNU治疗的398例术后病理证实为pT2~4NxM0期UTUC患者的临床数据。105例患者在RNU后90 d内接受吉西他滨加铂类辅助化疗纳入即刻化疗组,另外293例患者术后未接受化疗或未能在90 d内启动化疗而被纳入对照组。中位随访64(1~129)个月,监测化疗相关不良反应。使用Kaplan-Meier方法评估总生存期(OS)和癌症特异性生存期(CSS)。采用Cox回归风险模型评估预后因素和膀胱肿瘤复发的危险因素。结果 Cox多因素分析显示,性别、即刻辅助化疗、病理分级和N分期是独立的临床预后预测因素。Kaplan-Meier曲线显示,术后即刻化疗显著改善了UTUC患者的OS和CSS,尤其是淋巴结阳性患者。105例患者化疗期间未发生严重或致命的不良反应。60例患者术后出现膀胱肿瘤复发,其中术后即刻化疗组15例,对照组45例。多因素分析显示,伴发膀胱肿瘤是UTUC患者术后膀胱肿瘤复发的独立危险因素。结论 RNU术后90 d内开始的吉西他滨+铂类化疗方案显著改善了pT2~4NxM0期UTUC患者的OS和CSS,且无严重不良反应发生。伴发膀胱肿瘤的UTUC患者可能是RNU后膀胱肿瘤复发的高危人群之一。
-
关键词:
- 上尿路尿路上皮癌 /
- 化疗 /
- 根治性肾输尿管切除术
Abstract: Objective To determine whether patients with upper tract urothelial carcinoma(UTUC) with pT2-4NxM0 stage benefit from adjuvant chemotherapy started within 90 days after radical nephroureterectomy(RNU), and to identify risk factors for postoperative bladder recurrence.Methods The clinical data of 398 patients with pT2-4NxM0 stage UTUC who underwent RNU in the Affiliated Hospital of Xuzhou Medical University from 2011 to 2021 were retrospectively analyzed. One hundred and five patients who received gemcitabine and platinum-based adjuvant chemotherapy within 90 days after RNU were included in the immediate chemotherapy group, and 293 patients who did not receive postoperative chemotherapy or did not start chemotherapy within 90 days were included in the control group. The median follow-up period was 64(1-129) months, and chemotherapy-related adverse reactions were monitored. Overall survival(OS) and cancer-specific survival(CSS) were estimated using the Kaplan-Meier method. The Cox regression risk model was used to evaluate prognostic factors and risk factors for bladder tumor recurrence.Results Cox multivariate analysis showed that gender, immediate adjuvant chemotherapy, pathological grade, and N status were independent clinical predictors. Kaplan-Meier curves showed that immediate postoperative chemotherapy significantly improved OS and CSS in UTUC patients, especially in node-positive patients. No serious or fatal adverse reactions occurred in 105 patients during chemotherapy. Sixty patients had bladder tumor recurrences after surgery. Among them, 15 patients received immediate postoperative chemotherapy, and 45 patients were in the control group. Multivariate analysis showed that the presence of concomitant bladder tumors increased the risk of bladder recurrence in UTUC patients.Conclusion Gemcitabine plus platinum-based chemotherapy initiated within 90 days after RNU significantly improved OS and CSS in patients with pT2-4NxM0 stage UTUC without serious adverse events. One of the high-risk groups for bladder tumor recurrence following RNU may include UTUC patients with concomitant bladder tumors. -

-
表 1 患者临床特征
例(%) 项目 总体(398例) 对照组(293例) 即刻化疗组(105例) χ2 P 年龄 12.687 <0.001 ≥75岁 62(15.6) 57(19.5) 5(4.8) <75岁 336(84.4) 236(80.5) 100(95.2) 性别 0.253 0.615 男 265(66.6) 193(65.9) 72(68.6) 女 133(33.4) 100(34.1) 33(31.4) T分期 9.241 0.010 T2 123(30.9) 101(34.5) 22(21.0) T3 191(48.0) 130(44.4) 61(58.1) T4 84(21.1) 62(21.1) 22(20.9) N分期 14.296 <0.001 N0 271(68.1) 215(73.4) 56(53.3) N+ 127(31.9) 78(19.6) 49(46.7) 肿瘤部位 5.771 0.016 肾盂 218(54.8) 171(58.4) 47(44.8) 输尿管 180(45.2) 122(41.6) 58(55.2) 肾积水 229(57.5) 164(56.0) 65(62.0) 1.113 0.291 血尿 278(69.8) 205(70.0) 73(69.5) 0.007 0.933 腰痛 107(26.9) 79(27.0) 28(26.7) 0.003 0.953 伴发膀胱肿瘤 55(13.8) 35(12.0) 20(19.0) 3.274 0.070 肿瘤大小 0.247 0.619 ≥3 cm 175(44.0) 131(44.7) 44(41.9) <3 cm 223(56.0) 162(55.3) 61(58.1) 病理分级 14.391 <0.001 高级别 343(86.2) 241(82.3) 102(97.1) 低级别 55(13.8) 52(17.7) 3(2.9) 多态性 48(12.1) 32(10.9) 16(15.2) 1.358 0.244 表 2 OS的Cox单因素和多因素分析结果
项目 单因素分析 多因素分析 P HR 95%CI P HR 95%CI 性别 0.013 0.706 1.038~1.365 0.023 0.721 0.544~0.955 部位 0.438 1.111 0.851~1.450 即刻辅助化疗 <0.001 0.534 1.157~1.620 <0.001 0.381 0.269~0.542 伴发膀胱肿瘤 0.458 0.931 0.772~1.123 肾积水 0.078 1.275 0.974~1.669 血尿 0.065 0.766 0.576~1.017 腰痛 0.001 1.625 1.223~2.161 0.236 1.200 0.888~1.622 URS 0.006 0.591 0.406~0.861 0.025 0.642 0.436~0.947 N分期 <0.001 2.324 1.767~3.058 <0.001 2.650 1.976~3.553 多态性 0.327 1.213 0.825~1.782 病理分级 0.008 1.791 1.162~2.759 0.021 1.693 1.083~2.648 ≥3 cm 0.010 1.413 1.085~1.840 0.199 1.199 0.909~1.583 表 3 CSS的Cox单因素和多因素分析结果
项目 单因素分析 多因素分析 P HR 95%CI P HR 95%CI 性别 0.011 0.677 0.501~0.915 0.041 0.726 0.534~0.987 部位 0.314 1.164 0.866~1.565 即刻辅助化疗 <0.001 0.501 0.343~0.732 <0.001 0.355 0.240~0.526 伴发膀胱肿瘤 0.482 1.161 0.766~1.759 肾积水 0.095 1.291 0.956~1.742 血尿 0.050 0.732 0.535~1.000 0.306 0.846 0.615~1.165 腰痛 <0.001 1.697 1.245~2.312 0.351 1.170 0.841~1.628 URS 0.021 0.626 0.420~0.932 0.101 0.708 0.468~1.070 N分期 <0.001 2.472 1.836~3.329 <0.001 2.741 1.997~3.763 多态性 0.443 1.183 0.770~1.819 病理分级 0.005 2.125 1.252~3.607 0.020 1.912 1.106~3.304 ≥3 cm 0.006 1.507 1.124~2.018 0.118 1.279 0.939~1.742 表 4 即刻辅助化疗组患者的化疗不良反应
例(%) 不良反应 Ⅰ度 Ⅱ度 Ⅲ度 白细胞下降 46(43.8) 7(6.7) 3(2.9) 粒细胞下降 30(28.6) 5(47.6) 5(47.6) 血小板下降 22(21.0) 6(5.7) 3(2.9) 血红蛋白下降 38(36.2) 6(5.7) 3(2.9) 发热 13(12.4) 2(1.9) 0 过敏 1(0.9) 0 0 血尿素氮升高 26(24.8) 4(3.8) 0 血肌酐升高 33(31.4) 11(10.5) 1(0.9) 血尿 5(47.6) 0 0 蛋白尿 17(16.2) 8(7.6) 0 恶心/呕吐 42(40) 20(19.5) 6(5.7) 腹泻 8(7.6) 3(2.9) 0 便秘 11(10.5) 9(8.6) 0 谷丙转氨酶升高 18(17.1) 4(3.8) 0 碱性磷酸酶升高 16(15.2) 3(2.9) 0 胆红素升高 20(19.5) 7(6.7) 0 红斑/瘙痒 11(10.5) 14(13.3) 2(1.9) 脱发 21(20.0) 17(16.2) 2(1.9) 静脉炎 5(47.6) 1(0.9) 0 心功能障碍 4(3.8) 0 0 心包炎 3(2.9) 0 0 表 5 RNU术后膀胱肿瘤复发的危险因素分析
项目 单因素分析 多因素分析 P HR 95%CI P HR 95%CI 性别 0.734 0.890 0.454~1.744 年龄 0.571 0.911 0.659~1.259 部位 0.068 1.664 0.962~2.875 T分期 0.039 0.531 0.290~0.969 0.081 0.573 0.306~1.072 N分期 0.050 0.520 0.271~0.999 0.184 0.632 0.321~1.243 辅助化疗 0.456 1.251 0.694~2.256 伴发膀胱肿瘤 0.002 0.376 0.203~0.694 0.012 0.395 0.191~0.815 肾积水 0.020 0.503 0.282~0.897 0.101 0.603 0.330~1.103 URS 0.182 0.678 0.383~1.199 多态性 0.016 0.421 0.209~0.848 0.914 0.954 0.411~2.216 病理分级 0.136 0.495 0.197~1.247 -
[1] Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. doi: 10.3322/caac.21654
[2] Rouprêt M, Babjuk M, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update[J]. Eur Urol, 2021, 79(1): 62-79. doi: 10.1016/j.eururo.2020.05.042
[3] Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration[J]. Cancer, 2009, 115(6): 1224-1233. doi: 10.1002/cncr.24135
[4] Leow JJ, Chong KT, Chang SL, et al. Upper tract urothelial carcinoma: a different disease entity in terms of management[J]. ESMO Open, 2016, 1(6): e000126. doi: 10.1136/esmoopen-2016-000126
[5] Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder(EORTC 30994): an intergroup, open-label, randomised phase 3 trial[J]. Lancet Oncol, 2015, 16(1): 76-86. doi: 10.1016/S1470-2045(14)71160-X
[6] Raman JD, Lin YK, Kaag M, et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy[J]. Urol Oncol, 2014, 32(1): 47. e9-14. doi: 10.1016/j.urolonc.2013.06.015
[7] Seisen T, Granger B, Colin P, et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma[J]. Eur Urol, 2015, 67(6): 1122-1133. doi: 10.1016/j.eururo.2014.11.035
[8] 韩克威, 于芹超. 中性粒细胞与淋巴细胞比值对上尿路尿路上皮癌术后出现膀胱复发的预测价值[J]. 临床泌尿外科杂志, 2022, 37(12): 937-941. doi: 10.13201/j.issn.1001-1420.2022.12.010 http://lcmw.cbpt.cnki.net/WKC/WebPublication/paperDigest.aspx?paperID=8f1c74c7-4860-45ac-a4c2-f6671f2a5840
[9] Li YR, Yu KJ, Chang YH, et al. Predictors of Intravesical Recurrence After Radical Nephroureterectomy and Prognosis in Patients with Upper Tract Urothelial Carcinoma[J]. Cancer Manag Res, 2020, 12: 7439-7450. doi: 10.2147/CMAR.S261087
[10] Sheu ZL, Huang CP, Chang CH, et al. Tumor distribution affects bladder recurrence but not survival outcome of multifocal upper tract urothelial carcinoma treated with radical nephroureterectomy[J]. Sci Rep, 2021, 11(1): 19059. doi: 10.1038/s41598-021-98696-0
[11] Audenet F, Yates DR, Cussenot O, et al. The role of chemotherapy in the treatment of urothelial cell carcinoma of the upper urinary tract(UUT-UCC)[J]. Urol Oncol, 2013, 31(4): 407-413. doi: 10.1016/j.urolonc.2010.07.016
[12] 陈金虎, 方卫华, 梁朝朝. 上尿路尿路上皮癌临床治疗进展[J]. 临床泌尿外科杂志, 2021, 36(5): 415-420. doi: 10.13201/j.issn.1001-1420.2021.05.018 http://lcmw.cbpt.cnki.net/WKC/WebPublication/paperDigest.aspx?paperID=18f0ea33-3b78-43ee-99b3-22118d6d6c8c
[13] Kim DK, Kim JW, Jung HD, et al. Effects of Adjuvant Chemotherapy on Locally Advanced Upper Tract Urothelial Carcinoma: A Systematic Review and Meta-analysis[J]. Clin Genitourin Cancer, 2019, 17(6): e1193-e1202. doi: 10.1016/j.clgc.2019.08.010
[14] Necchi A, Lo Vullo S, Mariani L, et al. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration[J]. BJU Int, 2018, 121(2): 252-259. doi: 10.1111/bju.14020
[15] 赵子涵, 朱文洁, 王鑫, 等. 上尿路尿路上皮癌患者根治术后预后危险因素分析及风险分层模型构建[J]. 临床泌尿外科杂志, 2021, 36(9): 693-698. doi: 10.13201/j.issn.1001-1420.2021.09.004 http://lcmw.cbpt.cnki.net/WKC/WebPublication/advSearchPaperList.aspx?ys=&ist=&ns=&us=&ps=&pt=%E8%B7%AF%E4%B8%8A%E7%9A%AE%E7%99%8C%E6%82%A3%E8%80%85%E6%A0%B9%E6%B2%BB%E6%9C%AF%E5%90%8E%E9%A2%84%E5%90%8E%E5%8D%B1%E9%99%A9%E5%9B%A0%E7%B4%A0%E5%88%86%E6%9E%90%E5%8F%8A%E9%A3%8E%E9%99%A9&pks=&pc=&st=year&stp=&ds=&pcl=
[16] Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma(the POUT trial): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2020, 395(10232): 1268-1277. doi: 10.1016/S0140-6736(20)30415-3
[17] Hurel S, Rouprêt M, Seisen T, et al. Influence of preoperative factors on the oncologic outcome for upper urinary tract urothelial carcinoma after radical nephroureterectomy[J]. World J Urol, 2015, 33(3): 335-341. doi: 10.1007/s00345-014-1311-8
[18] Huang CC, Su YL, Luo HL, et al. Gender Is a Significant Prognostic Factor for Upper Tract Urothelial Carcinoma: A Large Hospital-Based Cancer Registry Study in an Endemic Area[J]. Front Oncol, 2019, 9: 157. doi: 10.3389/fonc.2019.00157
[19] Mohamad Al-Ali B, Madersbacher S, Zielonke N, et al. Impact of gender on tumor stage and survival of upper urinary tract urothelial cancer: A population-based study[J]. Wien Klin Wochenschr, 2017, 129(11-12): 385-390. doi: 10.1007/s00508-016-1088-4
[20] Habuchi T, Takahashi R, Yamada H, et al. Metachronous multifocal development of urothelial cancers by intraluminal seeding[J]. Lancet, 1993, 342(8879): 1087-1088. doi: 10.1016/0140-6736(93)92066-3
[21] Jones TD, Wang M, Eble JN, et al. Molecular evidence supporting field effect in urothelial carcinogenesis[J]. Clin Cancer Res, 2005, 11(18): 6512-6519. doi: 10.1158/1078-0432.CCR-05-0891
[22] Wang Q, Zhang T, Wu J, et al. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China[J]. BMC Urol, 2019, 19(1): 24. doi: 10.1186/s12894-019-0457-5
[23] Guo RQ, Hong P, Xiong GY, et al. Impact of ureteroscopy before radical nephroureterectomy for upper tract urothelial carcinomas on oncological outcomes: a meta-analysis[J]. BJU Int, 2018, 121(2): 184-193. doi: 10.1111/bju.14053
[24] Ouzzane A, Colin P, Xylinas E, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy[J]. Eur Urol, 2011, 60(6): 1258-1265. doi: 10.1016/j.eururo.2011.05.049
[25] Villa L, Haddad M, Capitanio U, et al. Which Patients with Upper Tract Urothelial Carcinoma Can be Safely Treated with Flexible Ureteroscopy with Holmium: YAG Laser Photoablation? Long-Term Results from a High Volume Institution[J]. J Urol, 2018, 199(1): 66-73. doi: 10.1016/j.juro.2017.07.088
[26] Galsky MD, JÁA A, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer(IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial[J]. Lancet, 2020, 395(10236): 1547-1557. doi: 10.1016/S0140-6736(20)30230-0
[27] Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer(KEYNOTE-052): a multicentre, single-arm, phase 2 study[J]. Lancet Oncol, 2017, 18(11): 1483-1492. doi: 10.1016/S1470-2045(17)30616-2
[28] Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma(IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial[J]. Lancet, 2018, 391(10122): 748-757. doi: 10.1016/S0140-6736(17)33297-X
[29] Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma(PURE-01): An Open-Label, Single-Arm, Phase Ⅱ Study[J]. J Clin Oncol, 2018, 36(34): 3353-3360. doi: 10.1200/JCO.18.01148
-




 下载:
下载: