Predictive model of prostate biopsy outcome based on MRI with different parameters and multidimensional clinical features
-
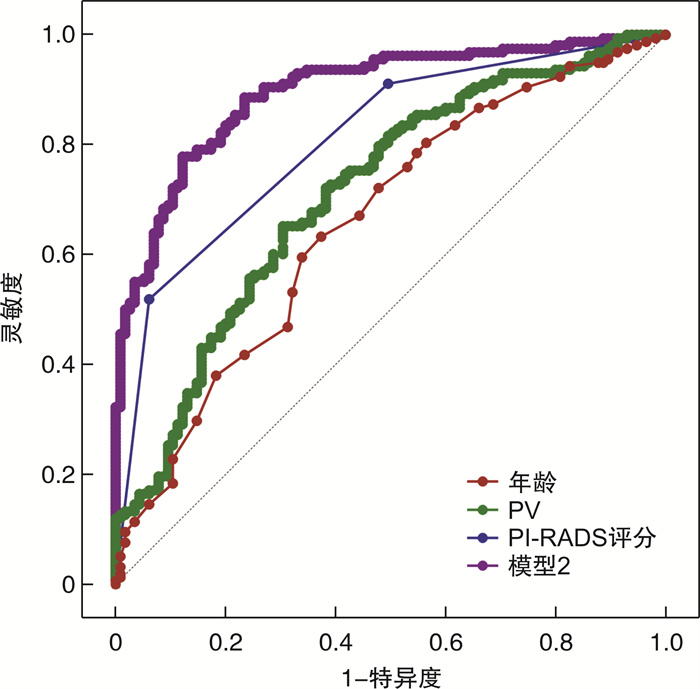
摘要: 目的 探索双参数MRI(bpMRI)和多参数MRI(mpMRI)检查联合多维度临床指标对前列腺穿刺活检结局的影响,构建相应的预测模型并评估其诊断价值。方法 回顾性纳入于2018年1月—2021年12月在南通大学第二附属医院泌尿外科行前列腺穿刺活检术的患者。结合年龄、总PSA(tPSA)、游离PSA(fPSA)、游离PSA/总PSA(f/tPSA)、前列腺特异性抗原密度(PASD)、前列腺体积(PV)、前列腺影像报告及数据系统(PI-RADS)评分等多维度临床指标,分别对行bpMRI和mpMRI检查患者穿刺活检出前列腺癌(PCa)进行单因素分析、多因素logistic回归分析,构建预测模型并绘制ROC曲线评估模型诊断价值。结果 ① bpMRI检查患者中,PCa组年龄、tPSA、PSAD、PV与PI-RADS评分显著高于非PCa组,f/tPSA则低于非PCa组; 多因素logistic回归分析发现,年龄、f/tPSA、PV与PI-RADS评分为预测PCa的独立危险因素; 基于bpMRI建立前列腺穿刺活检预测模型1,logitP=-10.52+0.10×年龄-7.21×f/tPSA-0.058×PV+1.70×PI-RADS评分; 受试者工作特征(ROC)曲线评估预测模型及独立危险因素对PCa的诊断价值,其中,年龄预测PCa的AUC为0.62(灵敏度为0.34,特异度为0.85),f/tPSA预测PCa的AUC为0.70(灵敏度为0.59,特异度为0.75),PV预测PCa的AUC为0.75(灵敏度为0.88,特异度为0.55),PI-RADS评分预测PCa的AUC为0.81(灵敏度为0.51,特异度为0.94),模型1预测PCa的AUC为0.91(灵敏度为0.84,特异度为0.86)。②mpMRI检查患者中,PCa组年龄、tPSA、PSAD、PV与PI-RADS评分显著高于非PCa组,f/tPSA则低于非PCa组; 多因素logistic回归分析发现,年龄、PV与PI-RADS评分为预测PCa的独立危险因素; 基于mpMRI建立前列腺穿刺活检预测模型2,logit P=-11.12+0.097×年龄-0.027×PV+1.48×PI-RADS评分; ROC曲线评估预测模型及独立危险因素对PCa的诊断价值,其中,年龄预测PCa的AUC为0.66(灵敏度为0.63,特异度为0.63),PV预测PCa的AUC为0.71(灵敏度为0.65,特异度为0.70),PI-RADS评分预测PCa的AUC为0.81(灵敏度为0.52,特异度为0.94),模型2预测PCa的AUC为0.90(灵敏度为0.77,特异度为0.88)。结论 多维度临床指标与bpMRI和mpMRI检查相结合均可提高PCa检出率; 其中,年龄、PV与PI-RADS可用于预测bpMRI和mpMRI检查患者前列腺穿刺活检结局,而f/tPSA仅对bpMRI检查患者前列腺穿刺活检结局有预测价值。Abstract: Objective To explore the influence of biparametricand multiparametric MRI(bpMRI and mpMRI) combined with multi-dimensional clinical indicators on the outcome of prostate biopsy, and to establish the corresponding predictive models, and evaluate their diagnostic value.Methods The patients who underwent prostate biopsy in the department of urology in the Second Affiliated Hospital of Nantong University from January 2018 to December 2021 were retrospectively included. Combined with the multi-dimensional clinical indicators of age, tPSA, fPSA, f/tPSA, PASD, PV, PI-RADS, single factor analysis and multiple factor logistic regression analysis were performed on the PCa detected by prostate biopsy in patients underging bpMRI and mpMRI, respectively. The predictive models were established and ROC curve was used to evaluate the diagnostic value of the models.Results ① In patients underging bpMRI, the age, tPSA, PSAD, PV and PI-RADS scores of PCa group were significantly higher than those of non-PCa group, while f/tPSA was lower than thatof non-PCa group. Multivariate logistic regression analysis showed that age, f/tPSA, PV and PI-RADS scores were independent risk factors for predicting PCa. The prostatebiopsy predictive model 1 was established based on bpMRI, logit P=-10.52+0.10×Age-7.21×f/tPSA-0.058×PV+1.70×PI-RADS. The ROC curve was used to evaluate the diagnostic value of predictive model and independent risk factors for PCa. The AUC of age for predicting PCa was 0.62(sensitivity: 0.34, specificity: 0.85), the AUC of f/tPSAfor predicting PCa was 0.70(sensitivity: 0.59, specificity: 0.75), the AUC of PV for predicting PCa was 0.75(sensitivity: 0.88, specificity: 0.55), and the AUC of PI-RADS scores for predicting PCa was 0.81(sensitivity: 0.51, specificity: 0.94), the AUC of model 1 for predicting PCa was 0.91(sensitivity: 0.84, specificity: 0.86). ②In patients underging mpMRI, the age, tPSA, PSAD, PV and PI-RADS scores of PCa group were significantly higher than those of non-PCa group, while f/tPSA was lower than thatof non-PCa group; Multivariate logistic regression analysis showed that age, PV and PI-RADS scores were independent risk factors for predicting PCa; The prostate biopsy predictive model 2 was established based on mpMRI, logit P=-11.12+0.097×Age-0.027×PV+1.48×PI-RADS. The ROC curve was used to evaluate the diagnostic value of predictive modeland independent risk factors for PCa. The AUC of age for predicting PCa was 0.66(sensitivity: 0.63, specificity: 0.63), the AUC of PV for predicting PCa was 0.71(sensitivity: 0.65, specificity: 0.70), the AUC of PI-RADS scores for predicting PCa was 0.81(sensitivity: 0.52, specificity: 0.94), and the AUC of model 2 for predicting PCa was 0.90(sensitivity: 0.77, specificity: 0.88).Conclusion The combination of multi-dimensional clinical indicators with bpMRI and mpMRI could improve the detection rate of PCa. Age, PV and PI-RADS scores could be used for predicting the outcome of prostate biopsy in patients underging bpMRI and mpMRI, while f/tPSA could only be used for predicting the outcome of prostate biopsy in patients underging bpMRI.
-
Key words:
- prostatic cancer /
- prostate biopsy /
- magnetic resonance imaging /
- diagnostic value /
- predictive model
-

-
表 1 bpMRI与mpMRI检查患者临床资料比较
例(%),X±S,M(P25,P75) 项目 bpMRI(298例) mpMRI(273例) t/Z/χ2 P值 年龄/岁 70.97±7.95 71.14±8.34 -0.26 0.80 tPSA/(ng/mL) 13.71(8.55,24.94) 12.02(7.36,25.15) -1.24 0.21 fPSA/(ng/mL) 1.75(1.05,3.11) 1.57(0.95,2.99) -1.61 0.11 f/tPSA 0.14±0.08 0.14±0.08 0.99 0.32 PSAD/(ng/mL2) 0.34(0.17,0.71) 0.32(0.18,0.63) -0.05 0.96 PV/mL 47.81±28.96 45.02±27.10 1.19 0.24 PI-RADS评分 2.40 0.30 3分 96(32) 72(26) 4分 115(39) 112(41) 5分 87(29) 89(33) PCa检出 2.45 0.12 非PCa 145(49) 115(42) PCa 153(51) 158(58) 表 2 基于bpMRI穿刺活检出PCa的单因素分析
X±S,M(P25,P75) 变量 非PCa组(145例) PCa组(153例) t/Z/χ2 P值 年龄/岁 69.00±8.12 72.83±7.34 -4.28 < 0.01 tPSA/(ng/mL) 12.21(7.78,17.72) 17.43(10.06,41.62) -4.69 < 0.01 tPSA<4 ng/mL 2(1) 5(3) 37.36 < 0.01 4 ng/mL≤tPSA≤10 ng/mL 54(37) 32(21) 10 ng/mL<tPSA≤20 ng/mL 68(47) 46(30) 20 ng/mL<tPSA≤100 ng/mL 21(14) 70(46) fPSA/(ng/mL) 1.69(1.11,2.55) 1.90(0.99,3.89) -1.23 0.22 f/tPSA 0.16±0.08 0.12±0.08 5.08 < 0.01 PSAD/(ng/mL2) 0.20(0.13,0.36) 0.54(0.29,1.21) -8.44 < 0.01 PV/mL 52.24(36.00,73.90) 33.85(24.40,42.09) -7.51 < 0.01 PI-RADS评分 97.32 < 0.01 3分 80(55) 16(10) 4分 56(39) 59(39) 5分 9(6) 78(51) 表 3 基于bpMRI穿刺活检出PCa的多因素logistic回归分析
变量 β OR 95%CI P值 年龄 0.10 1.11 1.05~1.16 < 0.01 tPSA 0.04 1.04 0.99~1.09 0.10 f/tPSA -7.21 < 0.01 0.00~0.06 < 0.01 PSAD -0.49 0.61 0.13~2.86 0.53 PV -0.06 0.94 0.92~0.97 < 0.01 PI-RADS评分 1.70 5.49 3.21~9.39 < 0.01 常量 -10.52 < 0.01 - < 0.01 表 4 基于mpMRI穿刺活检出PCa的单因素分析
例(%),X±S,M(P25,P75) 变量 非PCa组(115例) PCa组(158例) t/Z/χ2 P值 年龄/岁 68.46±8.40 73.09±7.75 -4.71 < 0.01 tPSA/(ng/mL) 9.29(6.70,16.41) 16.36(8.47,36.85) -4.96 < 0.01 tPSA<4 ng/mL 1(1) 6(4) 27.59 < 0.01 4 ng/mL≤tPSA≤10 ng/mL 62(54) 41(26) 10 ng/mL<tPSA≤20 ng/mL 30(26) 43(27) 20 ng/mL<tPSA≤100 ng/mL 22(19) 68(43) fPSA/(ng/mL) 1.43(0.89,2.21) 1.80(0.97,3.34) -2.14 0.03 f/tPSA 0.16±0.08 0.12±0.07 4.44 < 0.01 PSAD/(ng/mL2) 0.21(0.14,0.32) 0.49(0.25,1.07) -8.00 < 0.01 PV/mL 47.90(35.60,66.40) 33.22(24.18,43.59) -6.00 < 0.01 PI-RADS评分 86.76 < 0.01 3分 58(50) 14(9) 4分 50(44) 62(39) 5分 7(6) 82(52) 表 5 基于mpMRI穿刺活检出PCa的多因素logistic回归分析
变量 β OR 95%CI P值 年龄 0.10 1.10 1.05~1.15 < 0.01 tPSA < 0.01 1.01 0.79~1.28 0.97 f/tPSA -5.30 < 0.01 0.00~9.57 0.17 PSAD 1.63 5.09 0.75~34.68 0.10 PV -0.03 0.97 0.96~0.99 < 0.01 PI-RADS评分 1.48 4.38 2.52~7.62 < 0.01 常量 -11.12 < 0.01 — < 0.01 -
[1] Gandaglia G, Leni R, Bray F, et al. Epidemiology and Prevention of Prostate Cancer[J]. Eur Urol Oncol, 2021, 4(6): 877-892. doi: 10.1016/j.euo.2021.09.006
[2] Meng Y, Vetter JM, Parker AA, et al. Improved Detection of Clinically Significant Prostate Cancer With Software-assisted Systematic Biopsy Using MR/US Fusion in Patients With Negative Prostate MRI[J]. Urology, 2018, 120: 162-166. doi: 10.1016/j.urology.2018.06.020
[3] Welch HG, Albertsen PC. Reconsidering Prostate Cancer Mortality-The Future of PSA Screening[J]. N Engl J Med, 2020, 382(16): 1557-1563. doi: 10.1056/NEJMms1914228
[4] 贾伟, 吴波, 陈骞, 等. 前列腺健康指数及f/tPSA对于PSA"灰区"前列腺癌诊断价值比较的Meta分析[J/OL]. 中华腔镜泌尿外科杂志(电子版), 2021, 15(6): 478-483.
[5] Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2[J]. Eur Urol, 2016, 69(1): 16-40. doi: 10.1016/j.eururo.2015.08.052
[6] Choi MH, Kim CK, Lee YJ, et al. Prebiopsy Biparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2: A Multicenter Study[J]. AJR Am J Roentgenol, 2019, 212(4): 839-846. doi: 10.2214/AJR.18.20498
[7] 王永兴, 姜永光, 罗勇, 等. 前列腺癌患者年龄与临床病理特征的相关性[J]. 现代泌尿外科杂志, 2019, 24(8): 621-624. https://www.cnki.com.cn/Article/CJFDTOTAL-MNWK201908008.htm
[8] 高寒, 任冬, 闫九松, 等. 结合PI-RADSv2.1磁共振引导下PSAD和年龄建立预测前列腺癌风险的预测模型[J]. 川北医学院学报, 2021, 36(9): 1162-1166. doi: 10.3969/j.issn.1005-3697.2021.09.015
[9] 陶陶, 夏开国, 沈德贇, 等. 前列腺体积和炎性细胞浸润对前列腺穿刺活检阳性率的影响[J]. 中华男科学杂志, 2020, 26(5): 409-413. https://www.cnki.com.cn/Article/CJFDTOTAL-NKXB202005004.htm
[10] 卢振权, 罗兵锋, 李佩丰, 等. 基于多参数磁共振建立前列腺穿刺活检预测模型[J/OL]. 中华腔镜泌尿外科杂志(电子版), 2021, 15(4): 275-279.
[11] 梁震, 朱军, 康家旗, 等. 双参数磁共振PI-RADS联合PSA相关指标在首次前列腺穿刺活检中的诊断价值[J]. 中华泌尿外科杂志, 2019, 40(10): 768-773. https://cdmd.cnki.com.cn/Article/CDMD-10062-1021565429.htm
[12] Woo S, Suh CH, Kim SY, et al. Diagnostic Performance of Prostate Imaging Reporting and Data System Version 2 for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-analysis[J]. Eur Urol, 2017, 72(2): 177-188. doi: 10.1016/j.eururo.2017.01.042
[13] Mendhiratta N, Taneja SS, Rosenkrantz AB. The role of MRI in prostate cancer diagnosis and management[J]. Future Oncol, 2016, 12(21): 2431-2443. doi: 10.2217/fon-2016-0169
[14] Kim EH, Vemana G, Johnson MH, et al. Magnetic resonance imaging-targeted vs. conventional transrectal ultrasound-guided prostate biopsy: single-institution, matched cohort comparison[J]. Urol Oncol, 2015, 33(3): 109. e1-6. doi: 10.1016/j.urolonc.2014.09.004
[15] Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer(PROMIS): a paired validating confirmatory study[J]. Lancet, 2017, 389(10071): 815-822. doi: 10.1016/S0140-6736(16)32401-1
[16] Tamada T, Kido A, Yamamoto A, et al. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1[J]. J Magn Reson Imaging, 2021, 53(1): 283-291. doi: 10.1002/jmri.27283
[17] Boesen L, Nørgaard N, Løgager V, et al. Assessment of the Diagnostic Accuracy of Biparametric Magnetic Resonance Imaging for Prostate Cancer in Biopsy-Naive Men: The Biparametric MRI for Detection of Prostate Cancer(BIDOC)Study[J]. JAMA Netw Open, 2018, 1(2): e180219. doi: 10.1001/jamanetworkopen.2018.0219
-





 下载:
下载: