Pathologic and long-term outcomes of neoadjuvant chemotherapy for muscle-invasive bladder cancer: a single-center, 15-year real-world study
-
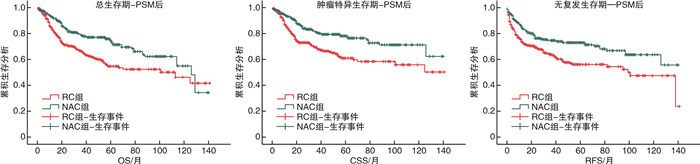
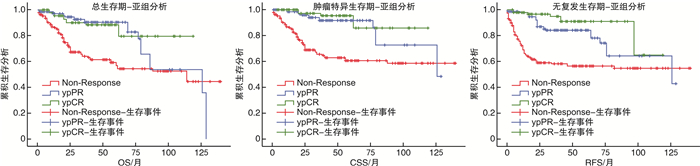
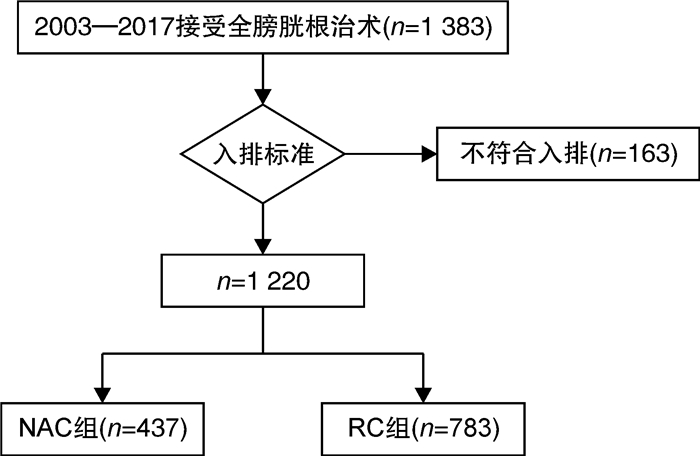
摘要: 目的 报道吉西他滨联合顺铂(GC)方案新辅助化疗联合全膀胱切除术治疗肌层浸润性膀胱癌(muscle-invasive bladder cancer,MIBC)的病理学与长期生存数据。方法 纳入2003—2017年上海交通大学医学院附属仁济医院临床诊断为MIBC、临床分期为cT2~4NanyM0,并行根治性全膀胱切除术的1 220例患者进行回顾性分析。按照患者术前是否接受新辅助化疗,分为单纯全膀胱组(RC组,783例)与新辅助联合全膀胱组(NAC组,437例)。对2组的队列特征、总生存期(overall survival,OS),肿瘤特异性生存期(cancer specific survival,CSS)、无复发生存期(recurrence free survival,RFS)、病理缓解率等进行比较。结果 NAC组患者的年龄与RC组相比显著降低;临床分期方面,RC组cT4a期患者比例高于NAC组(10.7% vs 5.7%,P=0.012),但2组cN分期比较差异无统计学意义(P=0.153)。NAC组达到完全病理缓解(complete pathological response,ypCR)的患者共99例(22.6%),达到部分病理缓解(partial pathological response,ypPR)的患者共142例(32.5%),总病理缓解(overall pathological response rate,ypRR)共241例(55.1%)。NAC组5年OS、CSS、RFS率分别为74.4%、76.6%、71.3%,10年OS、CSS、RFS率分别为48.1%、62.5%、55.7%,平均OS、CSS、RFS分别为97.6、108.6、99.2个月。RC组5年OS、CSS、RFS率分别为58.6%、64.1%、54.8%,10年OS、CSS、RFS率分别为46.9%、56.0%、32.7%,平均OS、CSS、RFS分别为89.6、97.5、83.2个月。经倾向性评分匹配后,NAC组的平均OS(P<0.001)、平均CSS(P<0.001)以及平均RFS(P<0.001)均显著优于RC组。结论 GC方案新辅助化疗可以显著改善MIBC患者的预后,使肿瘤通过化疗达到病理降期,延长OS、CSS、RFS。Abstract: Objective To assess the value of gemcitabine plus cisplatin(GC) neoadjuvant chemotherapy with radical cystectomy in the treatment of patients with muscle-invasive bladder cancer(MIBC).Methods This study included a retrospective analysis of a total of 1 220 cases diagnosed as MIBC(cT2-4NanyM0) in Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine from 2003 to 2017. According to whether received neoadjuvant chemotherapy before surgery, all patients were divided into a radical cystectomy group(RC group) and a neoadjuvant chemotherapy group(NAC group), and there were 783 cases in RC group and 437 cases in NAC group. The cohort characteristics, overall survival(OS), cancer specific survival(CSS), recurrence free survival(RFS), and pathological downstage rate of the two groups were compared.Results The age of patients in the NAC group was significantly younger than that in the RC group; In terms of clinical stage, the proportion of patients in the RC group with cT4a was higher than that in the NAC group(10.7% vs 5.7%, P=0.012), but no significant difference was found between the two groups in the cN stage(P=0.153). In the NAC group, 99 cases(22.6%) were found complete pathological response (ypCR), 142 cases(32.5%) were found ypPR and 241(55.1%) cases were found ypRR. In the NAC group, the 5-year OS, CSS, and RFS rates were 74.4%, 76.6%, and 71.3% respectively, the 10-year OS, CSS, and RFS rates were 48.1%, 62.5%, and 55.7% respectively, and the average duration of OS, CSS, and RFS were 97.6, 108.6, and 99.2 months. In the RC group, the 5-year OS, CSS, and RFS rates respectively were 58.6%, 64.1%, and 54.8%, the 10-year OS, CSS, and RFS rates were 46.9%, 56.0%, and 32.7%, and the average duration of OS, CSS, and RFS were 89.6, 97.5, and 83.2 months. After propensity score matching, NAC group was significantly better than the RC group in average OS(P=0.001), average CSS(P < 0.001), and average RFS(P < 0.001).Conclusion GC neoadjuvant chemotherapy can improve survival of the patients with MIBC, facilitate tumor downstage and increase the overall survival duration, the recurrence free survival duration and cancer specific survival duration.
-
Key words:
- bladder cancer /
- neoadjuvant chemotherapy /
- prognosis /
- long-term follow-up
-

-
表 1 研究队列临床病理参数比较
例(%),X±S 临床参数 RC组(783例) NAC组(437例) P值 男性 650(83.0) 340(77.8) 0.031 年龄/岁 67.4±7.7 62.7±10.0 <0.001 临床T分期 0.012 cT2 548(70.0) 319(73.0) cT3 151(19.3) 93(21.3) cT4a 84(10.7) 25(5.7) 临床N分期 0.153 cTanyN0 659(84.2) 381(87.2) cTanyN1~3 124(15.8) 56(12.8) 手术方式 0.788 开放 219(28.0) 128(29.3) 腹腔镜 308(39.3) 174(39.8) 机器人 256(32.7) 135(30.9) 病理T分期 <0.001 pT0 56(7.2) 108(24.7) pTa 9(1.1) 19(4.3) pT1 111(14.2) 107(24.5) pTis 20(2.6) 25(5.7) pT2 214(27.3) 56(12.8) pT3 266(34.0) 98(22.4) pT4 107(13.7) 24(5.5) 病理N分期a) 0.012 pTanyN0 498(80.6) 364(87.9) pTanyN1 66(10.7) 28(6.8) pTanyN2 39(6.3) 13(3.1) pTanyN3 15(2.4) 9(2.2) 合并原位癌 94(12.0) 52(11.9) 1.000 LVI 207(26.4) 60(13.7) <0.001 神经侵犯 91(11.6) 36(8.2) 0.078 切缘阳性 9(1.2) 4(0.9) 0.780 病理类型 鳞状分化 52(6.6) 18(4.1) 0.073 腺样分化 17(2.2) 12(2.7) 0.559 微乳头变异 20(2.6) 7(1.6) 0.317 神经内分泌分化 5(0.6) 2(0.5) 0.726 其他病理学变异 2(0.3) 0(0) 0.540 平均随访时间/月 30.5±29.3 35.4±25.2 <0.001 注:a)pN可分析1 032例,其中单纯RC队列618例,NAC+RC队列414例。 表 2 NAC组病理缓解率
例(%) 指标 NAC组(437例) GC方案(361例) 其他方案a)(76例) P值 ypCR 99(22.6) 89(24.6) 10(13.1) 0.034 ypPR 142(32.5) 116(32.1) 26(34.2) 0.787 ypRR 241(55.1) 205(55.9) 36(47.3) 0.162 注:a)包括GK方案61例,PC方案15例。 表 3 PSM后关键临床参数比较
例(%),X±S 临床参数 RC组(361例) NAC组(427例) P值 男性 300(83.1) 333(78.0) 0.08 年龄/岁 63.7±8.8 62.9±7.7 0.15 临床T分期 0.71 cT2 256(70.9) 314(73.5) cT3 85(23.5) 91(21.3) cT4a 20(5.5) 22(5.2) 临床N分期 0.83 cTanyN0 314(87.0) 374(87.6) cTanyN1~3 47(13.0) 53(12.4) -
[1] Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer[J]. Lancet, 2016, 388(10061): 2796-2810. doi: 10.1016/S0140-6736(16)30512-8
[2] Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017[J]. CA Cancer J Clin, 2017, 67(1): 7-30. doi: 10.3322/caac.21387
[3] Wu XR. Urothelial tumorigenesis: a tale of divergent pathways[J]. Nat Rev Cancer, 2005, 5(9): 713-725. doi: 10.1038/nrc1697
[4] Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer[J]. N Engl J Med, 2003, 349(9): 859-866. doi: 10.1056/NEJMoa022148
[5] Advanced Bladder Cancer(ABC)Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer(ABC)meta-analysis collaboration[J]. Eur Urol, 2005, 48(2): 202-205;discussion 205-206. doi: 10.1016/j.eururo.2005.04.006
[6] 曹明, 赵宏, 穆鑫, 等. 吉西他滨联合顺铂方案新辅助化疗联合全膀胱切除术治疗肌层浸润性膀胱癌的9年回顾性分析[J]. 中华泌尿外科杂志, 2014, 35(1): 49-53.
[7] Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis[J]. Lancet, 2003, 361(9373): 1927-1934. doi: 10.1016/S0140-6736(03)13580-5
[8] Burger M, Mulders P, Witjes W. Use of neoadjuvant chemotherapy for muscle-invasive bladder cancer is low among major European centres: results of a feasibility questionnaire[J]. Eur Urol, 2012, 61(5): 1070-1071. doi: 10.1016/j.eururo.2012.01.039
[9] Lobo N, Mount C, Omar K, et al. Landmarks in the treatment of muscle-invasive bladder cancer[J]. Nat Rev Urol, 2017, 14(9): 565-574. doi: 10.1038/nrurol.2017.82
[10] Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer[J]. Eur Urol, 2015, 67(2): 241-249. doi: 10.1016/j.eururo.2014.09.007
[11] Budman DR, Calabro A. Studies of synergistic and antagonistic combinations of conventional cytotoxic agents with the multiple eicosanoid pathway modulator LY 293111[J]. Anticancer Drugs, 2004, 15(9): 877-881. doi: 10.1097/00001813-200410000-00008
[12] van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma[J]. Cancer Discov, 2014, 4(10): 1140-1153. doi: 10.1158/2159-8290.CD-14-0623
[13] Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer[J]. Eur Urol, 2015, 68(6): 959-967. doi: 10.1016/j.eururo.2015.07.009
[14] Zhang R, Chen H, Xia J, et al. The pathological and clinical response of the luminal and basal subtypes of muscle-invasive bladder cancer to neoadjuvant cisplatin-based chemotherapy and radical cystectomy depend on the immunohistochemical classification system[J]. Eur Urol Suppl, 2017, 16(3): e303-e304. doi: 10.1016/S1569-9056(17)30244-0
[15] Yang Z, Zhang RY, Ge YX, et al. Somatic FGFR3 mutations distinguish a subgroup of muscle-invasive bladder cancers with response to neoadjuvant chemotherapy[J]. EBioMedicine, 2018, 35: 198-203. doi: 10.1016/j.ebiom.2018.06.011
[16] Miyamoto DT, Mouw KW, Feng FY, et al. Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer[J]. Lancet Oncol, 2018, 19(12): e683-e695. doi: 10.1016/S1470-2045(18)30693-4
[17] Font A, Taron M, Gago JL, et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer[J]. Ann Oncol, 2011, 22(1): 139-144. doi: 10.1093/annonc/mdq333
[18] Faltas BM, Prandi D, Tagawa ST, et al. Clonal evolution of chemotherapy-resistant urothelial carcinoma[J]. Nat Genet, 2016, 48(12): 1490-1499. doi: 10.1038/ng.3692
[19] Vlachostergios PJ, Faltas BM. Treatment resistance in urothelial carcinoma: an evolutionary perspective[J]. Nat Rev Clin Oncol, 2018, 15(8): 495-509. doi: 10.1038/s41571-018-0026-y
[20] Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma(PURE-01): an open-label, single-arm, phase Ⅱ study[J]. J Clin Oncol, 2018, 36(34): 3353-3360. doi: 10.1200/JCO.18.01148
-




 下载:
下载: