Efficacy and safety of Disitamab Vedotin compared with GC regimen combined with immunotherapy in the bladder-preserving treatment of bladder cancer
-
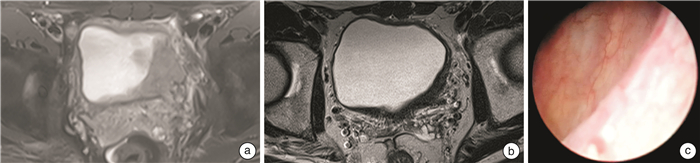
摘要: 目的 评估真实世界中维迪西妥单抗对比吉西他滨/顺铂(Gemcitabine/Cisplatin,GC)方案联合免疫治疗在膀胱癌保膀胱治疗中的疗效和安全性。方法 本研究是单中心真实世界的回顾性研究,连续纳入2023年3月—2024年3月于福建医科大学附属第一医院接受维迪西妥单抗联合免疫治疗和GC方案联合免疫治疗的33例膀胱癌患者的临床资料,分为A组(维迪西妥单抗联合免疫治疗)和B组(GC方案联合免疫治疗)。治疗后行影像学检查及二次根治性经尿道膀胱肿瘤电切术(transurethral resection of bladder tumor,TURBT)评估靶病灶变化情况。对比分析2组患者的病理完全缓解(pathological complete response,pCR)率、病理降期率、保膀胱率及预后情况,同时评估用药期间患者所发生的不良事件(adverse events,AEs)。结果 A组共纳入21例患者,其中16例选择保膀胱方案,5例选择膀胱根治切除术,中位随访时间9个月;B组共纳入12例患者,其中5例选择保膀胱方案,7例选择膀胱根治切除术,中位随访时间6个月。A组pCR率为52.4%(11/21),B组的pCR率为16.7%(2/12),差异有统计学意义(P=0.047);A组的病理降期率为81.0%(17/21),B组的病理降期率为58.3%(7/12),差异无统计学意义(P=0.159)。A组的保膀胱率为76.2%(16/21),B组的保膀胱率为41.7%(5/12),差异无统计学意义(P=0.055)。维迪西妥单抗联合免疫治疗常见的AEs包括高甘油三酯血症14例(66.7%)、血糖升高12例(57.1%)、肾功能减退10例(47.6%)等,其中3~4级AEs 3例(14.3%)。GC方案联合免疫治疗常见的AEs包括贫血7例(58.3%)、白细胞减少6例(50.0%)、肾功能减退5例(41.7%)、AST升高5例(41.7%)等,其中3~4级AEs 2例(16.7%)。结论 在膀胱癌治疗中,维迪西妥单抗联合免疫治疗较传统的GC方案联合免疫治疗具有更高的pCR率,病理降期率和保留膀胱率相仿,严重不良反应发生率低,且经治疗明显好转,在HER-2阴性的患者中亦有较好疗效。Abstract: Objective To evaluate the efficacy and safety of Disitamab Vedotin compared with Gemcitabine/Cisplatin(GC) regimen combined with immunotherapy in the bladder-preserving treatment of bladder cancer in the real-world setting.Methods This single-center retrospective study included 33 bladder cancer patients treated between March 2023 and March 2024 at the First Affiliated Hospital of Fujian Medical University. Patients received either Disitamab Vedotin combined with immunotherapy(Group A) or GC regimen combined with immunotherapy(Group B). Post-treatment imaging evaluations and secondary rigorous radical transurethral resection of bladder tumor(TURBT) at the original tumor site were performed to assess changes in target lesions. Pathological complete response(pCR), pathological downstaging rate, bladder preservation rate, and prognosis were compared between the two groups. Adverse events(AEs) during treatment were also evaluated.Results Group A included 21 patients, with 16 opting for bladder-preserving treatment and 5 undergoing radical cystectomy, with an average follow-up of 9 months. Group B included 12 patients, with 5 opting for bladder preservation and 7 undergoing radical cystectomy, with an average follow-up of 6 months. The pCR rate was 52.4%(11/21) in Group A and 16.7%(2/12) in Group B(P=0.047). Pathological downstaging rates were 81.0%(17/21) in Group A and 58.3%(7/12) in Group B(P=0.159). Bladder preservation rates were 76.2%(16/21) in Group A and 41.7%(5/12) in Group B(P=0.055). Common AEs during Disitamab Vedotin combined with immunotherapy included hypertriglyceridemia in 14 cases(66.7%), hyperglycemia in 12 cases(57.1%), and renal dysfunction in 10 cases(47.6%), with grade 3-4 AEs in 3 cases(14.3%). Common AEs during GC regimen combined with immunotherapy included anemia in 7 cases(58.3%), leukopenia in 6 cases(50.0%), renal dysfunction in 5 cases(41.7%), and AST elevation in 5 cases(41.7%), with grade 3-4 AEs in 2 cases(16.7%).Conclusion In bladder cancer treatment, Disitamab Vedotin combined with immunotherapy showed higher pCR rate compared to the traditional GC regimen combined with immunotherapy, similar pathological downstaging rate and bladder preservation success rate, lower incidence of severe adverse reactions, and significant therapeutic improvement, even in HER-2 negative patients.
-

-
表 1 2组患者基线特征比较
例(%) 项目 总例数(33例) A组(21例) B组(12例) P值 膀胱癌 0.320 MIBC 13(39.4) 7(33.3) 6(50.0) 高危NMIBC 14(42.4) 11(52.4) 3(25.0) 极高危NMIBC 6(18.2) 3(14.3) 3(25.0) 年龄 0.469 < 65岁 18(54.5) 10(47.6) 8(66.7) ≥65岁 15(45.5) 11(52.4) 4(33.3) 性别 1.000 男 25(75.8) 16(76.2) 9(75.0) 女 8(24.2) 5(23.8) 3(25.0) ECOG评分 0.719 ≤1 13(39.4) 9(42.9) 4(33.3) 2 20(60.6) 12(57.1) 8(66.7) HER-2表达水平 0.149 阴性 11(33.3) 5(23.8) 6(50.0) 阳性 22(66.7) 16(76.2) 6(50.0) 联合免疫治疗药物 0.819 特瑞普利单抗 16(48.5) 11(52.4) 5(41.7) 替雷利珠单抗 16(48.5) 9(42.9) 7(58.3) 帕博利珠单抗 1(3.0) 1(4.7) 0(0) 吸烟史 1.000 有 8(24.2) 5(23.8) 3(25.0) 无 25(75.8) 16(76.2) 9(75.0) 临床T分期 0.222 Tis、Ta、T1期 20(60.6) 14(66.7) 6(50.0) T2期 5(15.1) 4(19.0) 1(8.3) T3期 6(18.2) 3(14.3) 3(25.0) T4期 2(6.1) 0(0) 2(16.7) 病理分级 1.000 低级别 8(24.2) 5(23.8) 3(25.0) 高级别 25(75.8) 16(76.2) 9(75.0) 表 2 膀胱癌患者不同亚组维迪西妥单抗联合免疫治疗后ORR比较
例(%) 特征 例数 客观缓解 P值 特征 例数 客观缓解 P值 膀胱癌 0.363 联合免疫治疗药物 1.000 MIBC 7(33.3) 7(100.0) 特瑞普利单抗 11(52.4) 9(81.8) 高危NMIBC 11(52.4) 8(72.7) 替雷利珠单抗 9(42.9) 8(88.9) 极高危NMIBC 3(14.3) 3(100.0) 帕博利珠单抗 1(4.7) 1(100.0) 年龄 0.538 有无吸烟史 0.579 < 65岁 10(47.6) 9(90.0) 有 5(23.8) 4(80.0) ≥65岁 11(52.4) 9(81.8) 无 16(76.2) 14(87.5) 性别 0.579 临床T分期 1.000 男 16(76.2) 14(87.5) Tis、Ta、T1期 14(66.7) 11(78.6) 女 5(23.8) 4(80.0) T2期 4(19.0) 4(100.0) ECOG评分 0.388 T3期 3(14.3) 3(100.0) ≤1 9(42.9) 7(77.8) T4期 0(0) 0(0) 2 12(57.1) 11(91.7) 病理分级 0.579 HER-2表达水平 0.579 低级别 5(23.8) 4(80.0) 阴性 5(23.8) 4(80.0) 高级别 16(76.2) 14(87.5) 阳性 16(76.2) 14(87.5) 表 3 2组疗效比较
例(%) 组别 术后病理分期 pT0 pT1 pT2 pCR ≤pT1 A组(21例) 11(52.4) 6(28.6) 2(9.5) 11(52.4) 17(81.0) B组(12例) 2(16.7) 5(41.7) 3(25.0) 2(16.7) 7(58.3) P值 0.047 0.347 0.242 0.047 0.159 表 4 33例膀胱癌患者治疗相关AEs统计
例(%) AEs A组(21例) B组(12例) 合计(33例) 所有等级 3~4级 所有等级 3~4级 所有等级 3~4级 高甘油三酯血症 14(66.7) 0(0) 2(16.7) 0(0) 16(48.5) 0(0) 血糖升高 12(57.1) 1(4.7) 2(16.7) 0(0) 14(42.4) 1(3.0) 肾功能减退 10(47.6) 0(0) 5(41.7) 2(16.7) 15(45.5) 2(6.1) 贫血 8(38.1) 0(0) 7(58.3) 0(0) 15(45.5) 0(0) AST升高 8(38.1) 1(4.7) 5(41.7) 0(0) 13(39.4) 1(3.0) ALT升高 7(33.3) 1(4.7) 2(16.7) 0(0) 9(27.3) 1(3.0) 外周神经感觉异常 6(28.6) 0(0) 1(8.3) 0(0) 7(21.2) 0(0) 皮疹 5(23.8) 0(0) 1(8.3) 0(0) 6(18.2) 0(0) 脱发 4(19.0) 0(0) 0(0) 0(0) 4(12.1) 0(0) GGT升高 4(19.0) 0(0) 0(0) 0(0) 4(12.1) 0(0) 甲状腺功能减退 4(19.0) 0(0) 1(8.3) 0(0) 5(15.2) 0(0) 瘙痒 3(14.3) 0(0) 2(16.7) 0(0) 5(15.2) 0(0) 泌尿系统刺激症状 1(4.7) 0(0) 3(25.0) 0(0) 4(12.1) 0(0) 白细胞减少 1(4.7) 0(0) 6(50.0) 0(0) 7(21.2) 0(0) 中性粒细胞减少 1(4.7) 0(0) 4(33.3) 0(0) 5(15.2) 0(0) 结合胆红素升高 1(4.7) 0(0) 0(0) 0(0) 1(3.0) 0(0) 乏力 1(4.7) 0(0) 2(16.7) 0(0) 3(9.1) 0(0) 恶心 1(4.7) 0(0) 2(16.7) 0(0) 3(9.1) 0(0) 呕吐 1(4.7) 0(0) 2(16.7) 0(0) 3(9.1) 0(0) 腹胀 1(4.7) 0(0) 1(8.3) 0(0) 2(6.1) 0(0) 腹泻 1(4.7) 0(0) 0(0) 0(0) 1(3.0) 0(0) 下肢肿胀 1(4.7) 0(0) 0(0) 0(0) 1(3.0) 0(0) 免疫性结肠炎 1(4.7) 0(0) 0(0) 0(0) 1(3.0) 0(0) 注:GGT为谷氨酰转移酶。 -
[1] Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023[J]. CA Cancer J Clin, 2023, 73(1): 17-48. doi: 10.3322/caac.21763
[2] Yan M, Schwaederle M, Arguello D, et al. HER2 expression status in diverse cancers: review of results from 37, 992 patients[J]. Cancer Metastasis Rev, 2015, 34(1): 157-164. doi: 10.1007/s10555-015-9552-6
[3] Bolenz C, Shariat SF, Karakiewicz PI, et al. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder[J]. BJU Int, 2010, 106(8): 1216-1222. doi: 10.1111/j.1464-410X.2009.09190.x
[4] Sheng XN, Yan XQ, Wang L, et al. Open-label, multicenter, phase Ⅱ study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma[J]. Clin Cancer Res, 2021, 27(1): 43-51. doi: 10.1158/1078-0432.CCR-20-2488
[5] Bai XS, He WY, Yin HB, et al. Prognostic significance of HER2 status evaluation using immunohistochemistry in patients with urothelial carcinoma of the bladder: a retrospective single-center experience[J]. Exp Ther Med, 2022, 24(5): 704. doi: 10.3892/etm.2022.11640
[6] 中国临床肿瘤学会指南工作委员会. 免疫检查点抑制剂相关的毒性管理指南[M]. 北京: 人民卫生出版社, 2023: 204.
[7] 中国抗癌协会肿瘤病理专业委员会, 中国临床肿瘤学会尿路上皮癌专家委员会. 中国尿路上皮癌人表皮生长因子受体2检测临床病理专家共识[J]. 中华肿瘤杂志, 2021, 43(10): 1001-1006.
[8] Sheng XN, Wang L, He ZS, et al. Efficacy and safety of disitamab vedotin in patients with human epidermal growth factor receptor 2-positive locally advanced or metastatic urothelial carcinoma: a combined analysis of two phase Ⅱ clinical trials[J]. J Clin Oncol, 2024, 42(12): 1391-1402. doi: 10.1200/JCO.22.02912
[9] Tan X, Liu Z, Cai T, et al. Prognostic significance of HER2 expression in patients with Bacillus calmette-guérin-exposed non-muscle-invasive bladder cancer[J]. Eur Urol Oncol, 2024, 7(4): 760-769. doi: 10.1016/j.euo.2023.10.003
[10] Hu HL, Guo SZ, Huang SW, et al. MP16-09 TRUCE04: A Phase Ⅱ clinical trial of RC48 for high-risk non-muscle-invasive bladder cancer(HR-NMIBC)(NCT05495724)[J]. J Urol, 2024, 211(5S): e243.
[11] Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG(KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study[J]. Lancet Oncol, 2021, 22(7): 919-930. doi: 10.1016/S1470-2045(21)00147-9
[12] 周明, 杨潇, 曹强, 等. 新辅助化疗及联合免疫治疗在肌层浸润性膀胱癌中的疗效分析: 单中心真实世界回顾性研究[J]. 临床泌尿外科杂志, 2024, 39(6): 513-517. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2024.06.010
[13] Cahn DB, Handorf EA, Smaldone MC. Bladder extirpation vs preservation: the treatment debate[J]. JAMA Surg, 2018, 153(10): 889-890. doi: 10.1001/jamasurg.2018.1674
[14] Moran GW, Li G, Robins DJ, et al. Systematic review and meta-analysis on the efficacy of chemotherapy with transurethral resection of bladder tumors as definitive therapy for muscle invasive bladder cancer[J]. Bladder Cancer, 2017, 3(4): 245-258. doi: 10.3233/BLC-170134
[15] Galsky MD, Daneshmand S, Izadmehr S, et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial[J]. Nat Med, 2023, 29(11): 2825-2834. doi: 10.1038/s41591-023-02568-1
[16] Mazza P, Moran GW, Li G, et al. Conservative management following complete clinical response to neoadjuvant chemotherapy of muscle invasive bladder cancer: contemporary outcomes of a multi-institutional cohort study[J]. J Urol, 2018, 200(5): 1005-1013. doi: 10.1016/j.juro.2018.05.078
[17] Shi HZ, Zhang W, Bi XG, et al. Neoadjuvant chemotherapy-guided bladder-sparing treatment for muscle-invasive bladder cancer: results of a pilot phase Ⅱ study[J]. Cancer Res Treat, 2021, 53(4): 1156-1165. doi: 10.4143/crt.2020.1356
[18] Hussain SA, Porta N, Hall E, et al. Outcomes in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy followed by(chemo)radiotherapy in the BC2001 trial[J]. Eur Urol, 2021, 79(2): 307-315. doi: 10.1016/j.eururo.2020.11.036
[19] Herr H. Urologic principles define the standards for successful bladder preservation in muscle-invasive bladder cancer[J]. Eur Urol Focus, 2020, 6(4): 630-631. doi: 10.1016/j.euf.2019.12.003
[20] Wei YB, Zhang RC, Yu CB, et al. Disitamab vedotin in combination with immune checkpoint inhibitors for locally and locally advanced bladder urothelial carcinoma: a two-center's real-world study[J]. Front Pharmacol, 2023, 14: 1230395. doi: 10.3389/fphar.2023.1230395
[21] Xu JW, Zhang HQ, Zhang L, et al. Real-world effectiveness and safety of RC48-ADC alone or in combination with PD-1 inhibitors for patients with locally advanced or metastatic urothelial carcinoma: a multicenter, retrospective clinical study[J]. Cancer Med, 2023, 12(23): 21159-21171. doi: 10.1002/cam4.6680
-




 下载:
下载: