Causal relationship between type 2 diabetes and renal cell carcinoma based on a two-sample Mendelian randomization study
-
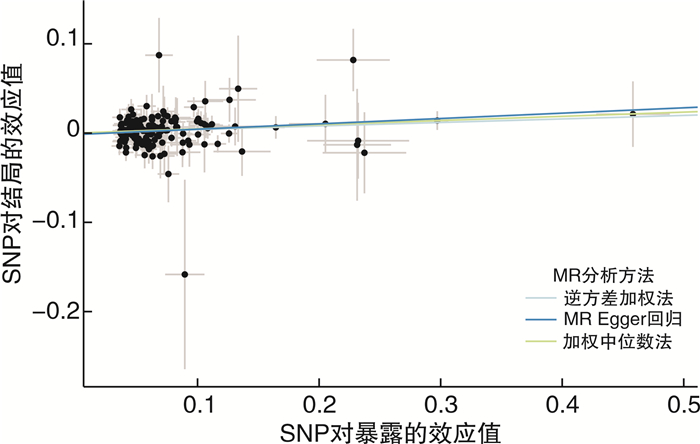
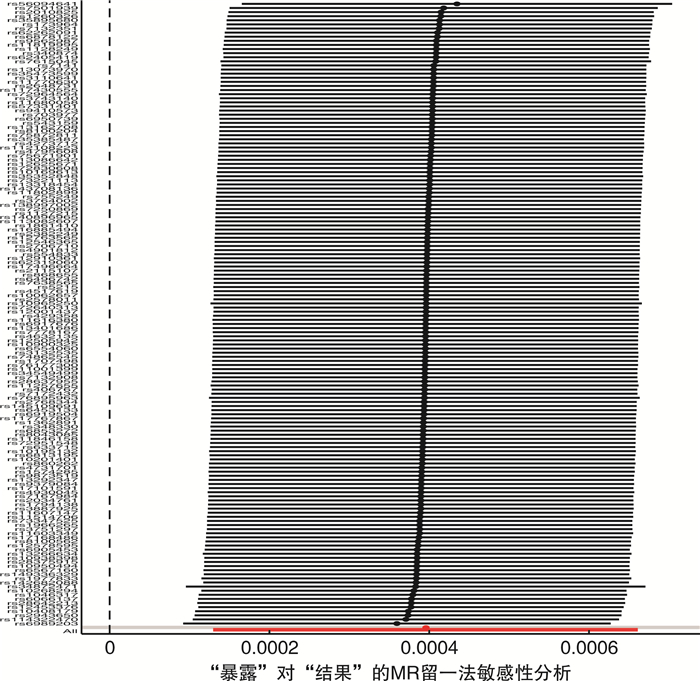
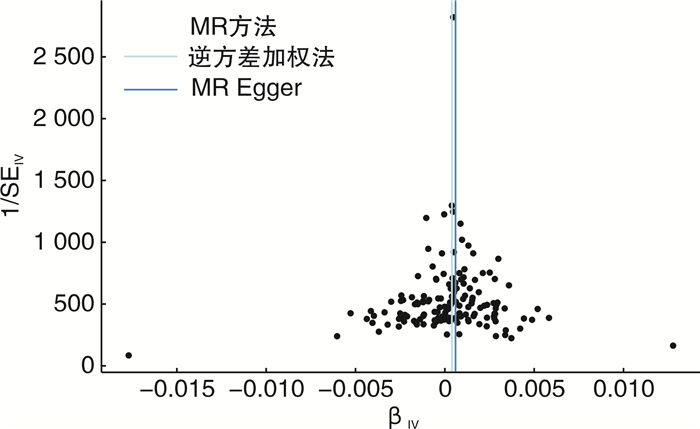
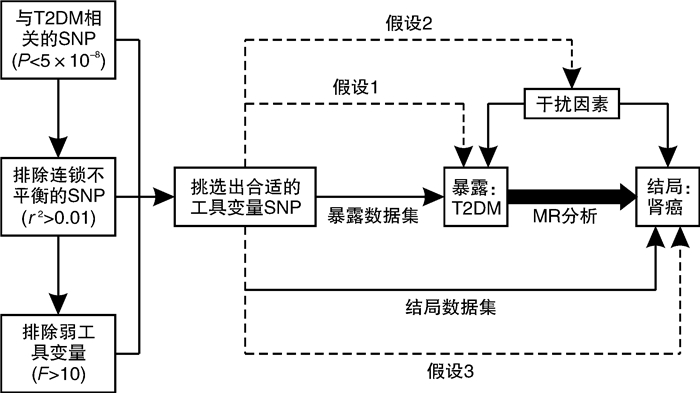
摘要: 目的 采用双样本孟德尔随机化探究2型糖尿病和肾癌的因果关系。方法 从GWAS Catalog数据库中筛选与2型糖尿病相关的单核苷酸多态性位点(P < 5×10-8)作为工具变量,用逆方差加权法作为主要的分析方法,并采用MR-Egger回归和加权中位数法作为补充。比值比(odd ratio,OR)值和95%置信区间(confidence interval,CI)用于评价2型糖尿病和肾癌之间是否存在因果关系。采用MR多效性检测水平多效性,采用留一法检测敏感性。结果 从UKB数据库男性和女性肾癌数据集中分别提取了152个SNP作为工具变量。MR-Egger回归、加权中位数和逆方差加权方法得到OR值和95%CI:女性1.27(95%CI:1.04~1.55),差异有统计学意义(P=0.003 5);男性1.04(95%CI:0.98~1.10),差异无统计学意义(P=0.33)。mr_pleiotropy_test显示水平多效性不显著。在进行留一敏感性分析后,没有观察到任何特定SNP位点对结果的显著影响,表明结果具有高度的稳定性和可靠性。结论 双样本孟德尔随机化分析显示2型糖尿病与女性肾癌可能存在因果关联。然而,需要进一步验证更大的样本量和来自不同种族背景的个人的数据,以加强研究结果。Abstract: Objective To investigate the potential causal relationship between type 2 diabetes and renal cell carcinoma by a two-sample Mendelian randomization method.Methods Single nucleotide polymorphisms(SNPs) associated with type 2 diabetes(P < 5×10-8) were screened from the GWAS Catalog database as instrumental variables. The inverse variance weighting method was used as the main analysis method, and MR-Egger regression and weighted median method were used to supplement the explanation. Odd ratio(OR) values and 95% confidence interval(CI) were used to evaluate the causal relationship between type 2 diabetes and renal cell carcinoma. Level pleiotropy was tested by mr_pleiotropy_test, and sensitivity was tested by leave-one-out method.Results From the UKB database male and female renal cell carcinoma data sets, 152 SNPs were extracted as instrumental variables, respectively. The MR-Egger regression, weighted median, and inverse variance weighting methods were used to obtain OR value and 95% CI: female 1.27(95%CI: 1.04-1.55) with statistical significance(P=0.003 5), male 1.04(95%CI: 0.98-1.10) without statistical significance(P=0.33). Mr_pleiotropy_test showed that horizontal pleiotropy was not statistically significant. After leave-one-out sensitivity analysis was performed, no significant effect of any specific SNP locus was observed, indicating a high degree of stability and reliability of the results.Conclusion The two-sample Mendelian randomization analysis showed a possible causal association between type 2 diabetes and renal cell carcinoma in women. However, further validation with larger sample sizes and data from individuals with different ethnic backgrounds is needed to strengthen the findings.
-

-
表 1 数据来源
数据库 数据集 描述 样本量/例 人群 包含SNP数量/个 病例来源 暴露数据集 Gwas Catalog GCST90018926 T2DM患者 490089 欧洲 24167560 根据ICD10的疾病分类招募参与者 结局数据集 UKB 20001_1034 男性肾癌患者
女性肾癌患者7119 3 780 英国 13791467 参与者的登记数据和自我报告的信息(由经验丰富的护士访谈验证) 表 2 女性肾癌患者中5种MR分析方法结果
方法 b SE P值 OR(95%CI) Q Q P值 IVW 0.24 0.10 0.003 5 1.27(1.04~1.55) 132.90 0.85 MR-Egger 0.42 0.03 0.037 1 1.05(1.44~1.61) 132.24 0.85 加权中位数 0.27 0.09 0.075 1 1.31(1.10~1.56) 加权模式 0.38 0.15 0.062 6 1.46(1.09~1.96) 简单模式 0.36 0.04 0.349 3 1.43(1.32~1.55) 表 3 男性肾癌患者中5种MR分析方法结果
方法 b SE P值 OR(95%CI) IVW 0.04 0.03 0.33 1.04(0.98~1.10) MR-Egger 0.19 0.05 0.65 1.21(1.10~1.33) 加权中位数 -0.01 0.04 0.11 0.99(0.92~1.07) 加权模式 -0.06 0.19 0.18 0.94(0.65~1.37) 简单模式 -0.11 0.11 0.17 0.99(0.80~1.23) -
[1] 施旭, 冯德超, 李登雄, 等. 代谢综合征和肾癌的研究进展[J]. 临床泌尿外科杂志, 2022, 37(9): 712-717. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2022.09.014
[2] Pruthi DK, Miller G, Ankerst DP, et al. Diabetes, obesity, and pathological upstaging in renal cell carcinoma: results from a large multi-institutional consortium[J]. J Urol, 2023, 210(5): 750-762. doi: 10.1097/JU.0000000000003650
[3] Vavallo A, Simone S, Lucarelli G, et al. Pre-existing type 2 diabetes mellitus is an independent risk factor for mortality and progression in patients with renal cell carcinoma[J]. Medicine(Baltimore), 2014, 93(27): e183.
[4] Wang S, Lo Galbo MD, Blair C, et al. Diabetes and kidney cancer risk among post-menopausal women: the Iowa women's health study[J]. Maturitas, 2021, 143: 190-196. doi: 10.1016/j.maturitas.2020.07.010
[5] Gagnon E, Daghlas I, Zagkos L, et al. Mendelian randomization applied to neurology: promises and challenges[J]. Neurology, 2024, 102(4): e209128. doi: 10.1212/WNL.0000000000209128
[6] Levin MG, Burgess S. Mendelian randomization as a tool for cardiovascular research: a review[J]. JAMA Cardiol, 2024, 9(1): 79-89. doi: 10.1001/jamacardio.2023.4115
[7] Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization[J]. Stat Med, 2021, 40(25): 5434-5452. doi: 10.1002/sim.9133
[8] Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization[J]. Genet Epidemiol, 2023, 47(4): 314-331. doi: 10.1002/gepi.22522
[9] Lin ZT, Pan I, Pan W. A practical problem with Egger regression in Mendelian randomization[J]. PLoS Genet, 2022, 18(5): e1010166. doi: 10.1371/journal.pgen.1010166
[10] Lu ZC, Chen YS, Tang ZC, et al. Basal metabolic rate and the risk of urolithiasis: a two-sample Mendelian randomization study[J]. World J Urol, 2024, 42(1): 235. doi: 10.1007/s00345-024-04946-x
[11] Li N, Wang Y, Wei P, et al. Causal effects of specific gut microbiota on chronic kidney diseases and renal function-a two-sample Mendelian randomization study[J]. Nutrients, 2023, 15(2): 360. doi: 10.3390/nu15020360
[12] Yang WW, Yang YJ, He L, et al. Dietary factors and risk for asthma: a Mendelian randomization analysis[J]. Front Immunol, 2023, 14: 1126457. doi: 10.3389/fimmu.2023.1126457
[13] Pallauf M, Ged Y, Singla N. Molecular differences in renal cell carcinoma between males and females[J]. World J Urol, 2023, 41(7): 1727-1739. doi: 10.1007/s00345-023-04347-6
[14] Habib SL, Prihoda TJ, Luna M, et al. Diabetes and risk of renal cell carcinoma[J]. J Cancer, 2012, 3: 42-48. doi: 10.7150/jca.3718
[15] Peired AJ, Lazzeri E, Guzzi F, et al. From kidney injury to kidney cancer[J]. Kidney Int, 2021, 100(1): 55-66. doi: 10.1016/j.kint.2021.03.011
[16] Wang L, Xu R, Kaelber DC, et al. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes[J]. JAMA Netw Open, 2024, 7(7): e2421305. doi: 10.1001/jamanetworkopen.2024.21305
[17] Ghareghomi S, Arghavani P, Mahdavi M, et al. Hyperglycemia-driven signaling bridges between diabetes and cancer[J]. Biochem Pharmacol, 2024, 229: 116450. doi: 10.1016/j.bcp.2024.116450
[18] Das A, Reis F. mTOR signaling: new insights into cancer, cardiovascular diseases, diabetes and aging[J]. Int J Mol Sci, 2023, 24(17): 13628. doi: 10.3390/ijms241713628
[19] Liu BM, Paudel S, Flowers WL, et al. Uterine histotroph and conceptus development: Ⅲ. adrenomedullin stimulates proliferation, migration and adhesion of porcine trophectoderm cells via AKT-TSC2-MTOR cell signaling pathway[J]. Amino Acids, 2023, 55(6): 743-756. doi: 10.1007/s00726-023-03265-6
[20] Zhan SZ, Bai XJ, Zhao YQ, et al. TGFBI promotes proliferation and epithelial-mesenchymal transition in renal cell carcinoma through PI3K/AKT/mTOR/HIF-1α pathway[J]. Cancer Cell Int, 2024, 24(1): 265. doi: 10.1186/s12935-024-03454-7
[21] Garczorz W, Kosowska A, Francuz T. Antidiabetic drugs in breast cancer patients[J]. Cancers(Basel), 2024, 16(2): 299.
[22] Ahmmed R, Hossen MB, Ajadee A, et al. Bioinformatics analysis to disclose shared molecular mechanisms between type-2 diabetes and clear-cell renal-cell carcinoma, and therapeutic indications[J]. Sci Rep, 2024, 14(1): 19133. doi: 10.1038/s41598-024-69302-w
-




 下载:
下载: