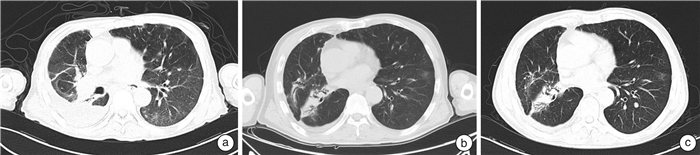
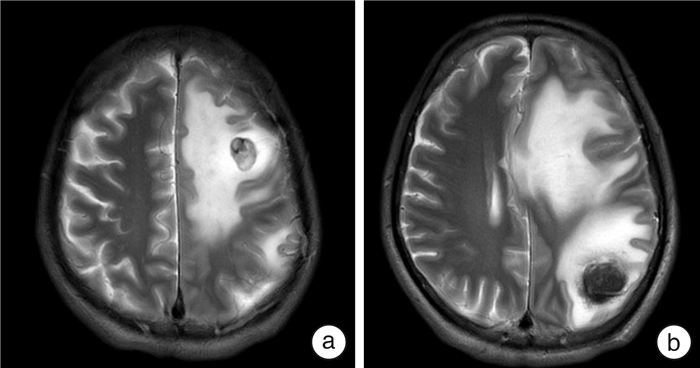
Summary of single-center experience in the treatment of metastatic renal cancer based on immune checkpoint inhibitors
-
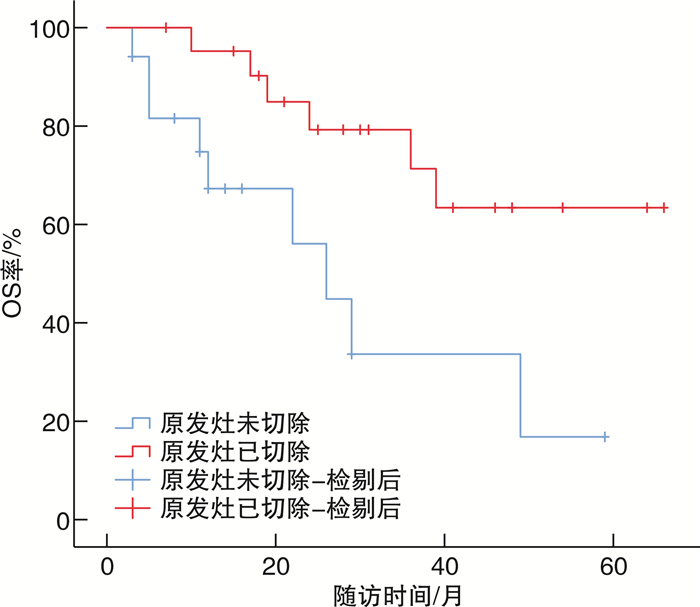
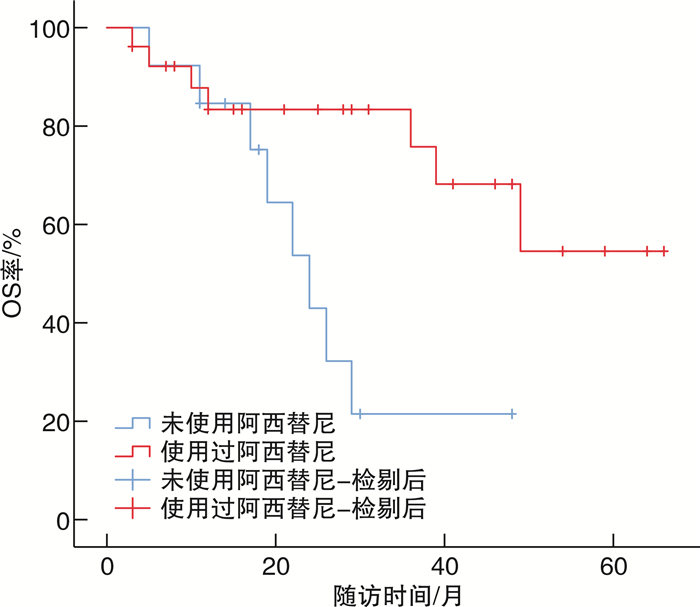
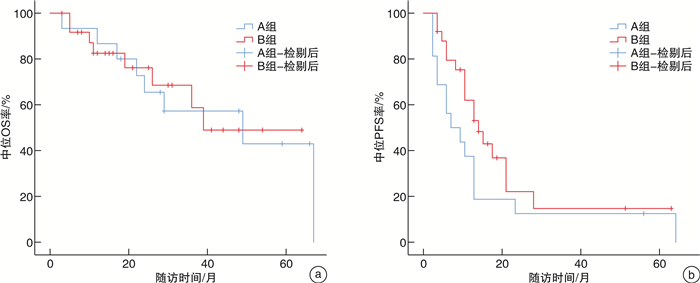
摘要: 目的 总结基于免疫检测点抑制剂在转移性肾癌(metastatic renal cell carcinoma,mRCC)治疗中的单中心治疗经验。方法 回顾性分析2019年7月—2020年7月厦门大学附属第一医院收治61例应用程序性死亡蛋白-1(programmed cell death protein-1,PD-1)单抗治疗的晚期mRCC患者的临床资料。根据一线治疗方法将患者分为2组。一线单用靶向治疗24例(A组),男18例,女6例;透明细胞癌20例,非透明细胞癌4例;行原发灶切除术10例。一线靶向联合免疫治疗37例(B组),男29例,女8例;透明细胞癌30例,非透明细胞癌7例;行原发灶切除18例。2组患者一般资料比较均差异无统计学意义(P>0.05)。靶向治疗药物包括阿昔替尼、舒尼替尼、培唑帕尼、依维莫司、索拉非尼、仑伐替尼,免疫治疗药物包括替雷利珠单抗。一线单用靶向治疗患者在疾病进展后更换为免疫联合靶向治疗或其他靶向药物治疗方案,一线靶向联合免疫治疗患者在疾病进展或不能耐受后根据患者情况更换其他靶免方案或单纯靶向药物继续治疗;分析mRCC患者总体人群和各亚组的客观缓解率(objective response rate,ORR)、无进展生存期(progression-free survival,PFS)、总生存时间(overall survival,OS)等。结果 61例中位随访时间24(12,43)个月,A、B组的中位OS分别为39(26,未达)个月、49(22,67)个月,中位PFS分别为6(3,11)个月、13(9,18)个月;A、B组的2年OS率分别为66%、76%,5年OS率分别为38%、49%。在靶免联合基础上既往接受过原发灶切除手术患者预后优于原发灶未切除患者(P=0.008),前者中位OS未达到,后者中位OS为24(19,29)个月。单因素分析结果 显示,OS与原发灶切除、使用阿昔替尼相关(P < 0.01),相较于未切除原发灶的患者,原发灶切除患者死亡风险下降约70%(HR=0.39,95%CI:0.17~0.89,P=0.026),而治疗过程中应用阿昔替尼的患者死亡风险下降约75%(HR=0.25,95%CI:0.06~0.98,P=0.047)。8例一线治疗单用靶向治疗药物患者出现3~4级不良反应,11例靶向联合免疫治疗患者出现3~4级不良反应。主要3~4级不良反应为甲状腺功能减退、白细胞减少、肝功能损害、垂体功能减退、严重腹泻、肾上腺皮质功能减退。结论 针对mRCC患者早期靶向联合免疫治疗方案在PFS和OS方面要优于一线靶向治疗方案,原发灶切除、使用阿昔替尼可能有利于改善患者预后。Abstract: Objective To summarize the experience of diagnosis and treatment of metastatic renal cell carcinoma(mRCC) patients treated with targeted drugs at a single center.Methods The clinical data of 61 mRCC patients treated with programmed cell death protein-1(PD-1) monoclonal antibody at First Affiliated Hospital of Xiamen University from July 2019 to July 2020 were retrospectively analyzed. According to the first-line treatment method, the patients were divided into two groups. Group A(24 cases) received single-agent targeted therapy as the first-line treatment, including 18 males and 6 females, 20 clear cell carcinoma cases and 4 non-clear cell carcinoma cases; 10 cases underwent nephrectomy. Group B(37 cases) received targeted combined immunotherapy as the first-line treatment, including 29 males and 8 females, 30 clear cell carcinoma cases and 7 non-clear cell carcinoma cases, 18 cases underwent nephrectomy. There were no statistically significant differences in the general data of the two groups(P>0.05). The targeted therapy drugs included axitinib, sunitinib, pazopanib, everolimus, sorafenib and lenvatinib. The immunotherapy drug included tislelizumab. Patients who received single-agent targeted therapy in the first line were switched to immune-combined targeted therapy or other targeted drug regimens after disease progression, while patients who received first-line targeted therapy combined with immunotherapy were switched to other targeted therapy regimens or continued with pure targeted drug therapy after disease progression or intolerance. The overall population and subgroups of patients with mRCC were analyzed for objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) etc.Results Among the 61 patients, the median follow-up time was 24(12, 43) months. The median OS was 39(26, not reached) months for group A and 49(22, 67) months for group B, and the median PFS was 6(3, 11) months for group A and 13(9, 18) months for group B. The 2-year survival rate were 66% for group A and 76% for group B, and the 5-year survival rate were 38% for group A and 49% for group B. Patients who had undergone primary tumor resection prior to immunotherapy combined targeted therapy had better prognoses than those who had not(P=0.008), with a median OS that had not yet been reached for the former and 24(19, 29) months for the latter. The results of univariate analysis showed that OS was associated with primary tumor resection and the use of axitinib(P < 0.01). Compared with patients who did not undergo primary tumor resection, the risk of death for patients who underwent primary tumor resection decreased by about 70%(HR=0.39, 95%CI: 0.17-0.89, P=0.026), while the risk of death for patients who received axitinib during treatment decreased by about 75%(HR=0.25, 95%CI: 0.06-0.98, P=0.047). Three to four grade adverse reactions were observed in 8 patients receiving targeted therapy in the first line, and 3 to 4 grade adverse reactions were observed in 11 patients receiving targeted therapy combined with immunotherapy. The main 3 to 4 grade adverse reactions were hypothyroidism, leukopenia, liver function damage, hypopituitarism, severe diarrhea, and adrenocortical insufficiency.Conclusion The early targeted combined immunotherapy regimen for mRCC patients is superior to the monotherapy in PFS and OS. Primary tumor resection and the use of axitinib may be beneficial to prognosis.
-

-
表 1 晚期肾癌患者一般资料
例 项目 A组(24例) B组(37例) 性别 男 18 29 女 6 8 年龄 < 60岁 7 14 ≥60岁 17 23 病理类型 透明细胞癌 20 30 非透明细胞癌 4 7 IMDC评分 低危 2 4 中危 17 24 高危 5 9 原发灶切除 10 18 表 2 转移性肾癌患者临床病理资料与预后的亚组分析
项目 中位PFS/月 P值 中位OS/月 P值 年龄 0.31 0.65 < 60岁 9 40 ≥60岁 11 49 病理类型 0.36 0.05 透明细胞癌 15 未达 非透明细胞癌 10 49 转移部位 0.21 0.02 肝/脑 9 29 肺/骨/其他部位 13 49 原发灶切除 0.28 0.01 是 11 未达 否 11 24 靶向药物为阿昔替尼 0.85 0.01 是 11 未达 否 9 26 -
[1] Coppin C, Porzsolt F, Awa A, et al. Immunotherapy for advanced renal cell cancer[J]. Cochrane Database Syst Rev, 2005(1): CD001425.
[2] Atzpodien J, Kirchner H, Jonas U, et al. Interleukin-2-and interferon Alfa-2a-based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the German cooperative renal carcinoma chemoimmunotherapy group(DGCIN)[J]. J Clin Oncol, 2004, 22(7): 1188-1194. doi: 10.1200/JCO.2004.06.155
[3] Kumar R, Kapoor A. Current management of metastatic renal cell carcinoma: evolving new therapies[J]. Curr Opin Support Palliat Care, 2017, 11(3): 231-237. doi: 10.1097/SPC.0000000000000277
[4] Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial[J]. Lancet Oncol, 2019, 20(10): 1370-1385. doi: 10.1016/S1470-2045(19)30413-9
[5] 王荀, 郑军华, 翟炜. EAU 2024热点速递: 肾细胞癌的临床研究进展[J]. 临床泌尿外科杂志, 2024, 39(6): 544-546, 550. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2024.06.016
[6] Desai J, Deva S, Lee JS, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors[J]. J Immunother Cancer, 2020, 8(1): e000453. doi: 10.1136/jitc-2019-000453
[7] Ruiz-Morales JM, Swierkowski M, Wells JC, et al. First-line sunitinib versus pazopanib in metastatic renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium[J]. Eur J Cancer, 2016, 65: 102-108. doi: 10.1016/j.ejca.2016.06.016
[8] Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study[J]. J Immunother Cancer, 2018, 6(1): 109. doi: 10.1186/s40425-018-0420-0
[9] Chowdhury S, Infante JR, Hawkins R, et al. A phase Ⅰ/Ⅱ study to assess the safety and efficacy of pazopanib and pembrolizumab combination therapy in patients with advanced renal cell carcinoma[J]. Clin Genitourin Cancer, 2021, 19(5): 434-446. doi: 10.1016/j.clgc.2021.04.007
[10] Lu XL, Gu WJ, Shi GH, et al. Pazopanib together with 6-8 cycles of sintilimab followed by single use of pazopanib in the second-line treatment of advanced renal cell carcinoma[J]. Transl Androl Urol, 2021, 10(5): 2078-2083. doi: 10.21037/tau-21-338
[11] Lalani AA, McGregor BA, Albiges L, et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: current paradigms, use of immunotherapy, and future directions[J]. Eur Urol, 2019, 75(1): 100-110. doi: 10.1016/j.eururo.2018.10.010
[12] Mattei J, da Silva RD, Sehrt D, et al. Targeted therapy in metastatic renal carcinoma[J]. Cancer Lett, 2014, 343(2): 156-160. doi: 10.1016/j.canlet.2013.09.038
[13] Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma[J]. N Engl J Med, 2019, 380(12): 1116-1127. doi: 10.1056/NEJMoa1816714
[14] Osanto S, van der Hulle T. Cabozantinib in the treatment of advanced renal cell carcinoma in adults following prior vascular endothelial growth factor targeted therapy: clinical trial evidence and experience[J]. Ther Adv Urol, 2018, 10(3): 109-123. doi: 10.1177/1756287217748867
[15] Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma[J]. N Engl J Med, 2018, 378(14): 1277-1290. doi: 10.1056/NEJMoa1712126
[16] Flippot R, Escudier B, Albiges L. Immune checkpoint inhibitors: toward new paradigms in renal cell carcinoma[J]. Drugs, 2018, 78(14): 1443-1457. doi: 10.1007/s40265-018-0970-y
[17] Xu WX, Atkins MB, McDermott DF. Checkpoint inhibitor immunotherapy in kidney cancer[J]. Nat Rev Urol, 2020, 17(3): 137-150. doi: 10.1038/s41585-020-0282-3
[18] Herbst RS, Garon EB, Kim DW, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study[J]. J Clin Oncol, 2020, 38(14): 1580-1590. doi: 10.1200/JCO.19.02446
-




 下载:
下载: