Recent developments in non-invasive localization diagnosis of primary aldosteronism
-
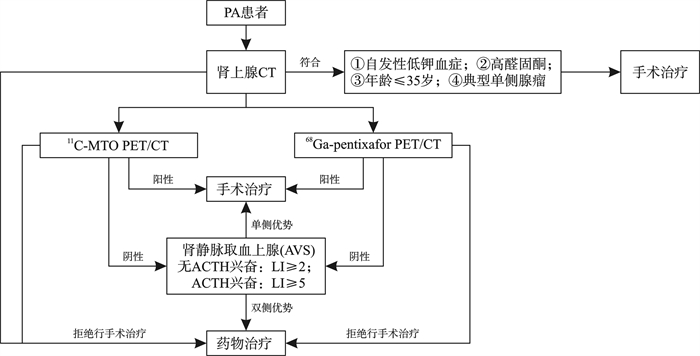
摘要: 原发性醛固酮增多症(primary aldosteronism,PA)是继发性高血压最常见的病因之一。高血压对靶器官的直接损害可能导致肾脏和心血管系统等不良后果。准确的定位诊断对于选择适当的治疗方案至关重要。目前临床上广泛采用肾上腺静脉采血(adrenal vein sampling,AVS)作为PA定位诊断的最佳方式。然而,AVS存在相应的局限性,如有创性、价格昂贵、技术要求高等。因此无创定位诊断在PA的诊疗过程中有望得到更广泛的应用。本综述将探讨无创定位诊断在PA诊断、治疗管理和预后评估中的应用。Abstract: Primary aldosteronism (PA) stands as one of the most prevalent etiologies of secondary hypertension. The direct impact of hypertension on target organs can result in adverse outcomes within the renal and cardiovascular systems. Accurate localization diagnosis plays a pivotal role in determining appropriate treatment modalities. Currently, adrenal vein sampling (AVS) is widely adopted as the gold standard for localizing PA. However, AVS is encumbered by inherent limitations including invasiveness, high cost, and technical intricacies. Consequently, non-invasive localization diagnosis is poised to gain broader utility in the diagnosis and therapy of PA. This review aims to delve into the application of non-invasive localization diagnosis in the diagnostic, therapeutic management, and prognostic evaluation of PA.
-
Key words:
- primary aldosteronism /
- subtyping diagnosis /
- nuclear molecular imaging
-

-
[1] Turcu AF, Yang J, Vaidya A. Primary aldosteronism-a multidimensional syndrome[J]. Nat Rev Endocrinol, 2022, 18(11): 665-682. doi: 10.1038/s41574-022-00730-2
[2] Kocjan T, Vidmar G, Popović P, et al. Validation of three novel clinical prediction tools for primary aldosteronism subtyping[J]. Endocr Connect, 2022, 11(5): e210532.
[3] 方晨, 戴军, 赵菊平, 等. 基于CT影像的手术决策治疗单侧醛固酮腺瘤疗效研究[J]. 临床泌尿外科杂志, 2023, 38(9): 656-661.
[4] Shidlovskyi VO, Shidlovskyi OV, Sheremet M, et al. Laboratory diagnostics of primary hyperaldosteronism and its peculiarities(literature review)[J]. J Med Life, 2019, 12(3): 215-220. doi: 10.25122/jml-2019-0073
[5] Zhou YQ, Wang D, Jiang LC, et al. Diagnostic accuracy of adrenal imaging for subtype diagnosis in primary aldosteronism: systematic review and meta-analysis[J]. BMJ Open, 2020, 10(12): e038489. doi: 10.1136/bmjopen-2020-038489
[6] Pilz S, Kocjan T, Theiler-Schwetz V, et al. Primary aldosteronism 2.0: an update for clinicians on diagnosis and treatment[J]. Pol Arch Intern Med, 2023, 133(10): 16585. http://openurl.ebsco.com/contentitem/doi:10.20452%2Fpamw.16585?sid=ebsco:plink:crawler&id=ebsco:doi:10.20452%2Fpamw.16585
[7] Xu ZX, Yang J, Hu JB, et al. Primary aldosteronism in patients in China with recently detected hypertension[J]. J Am Coll Cardiol, 2020, 75(16): 1913-1922. doi: 10.1016/j.jacc.2020.02.052
[8] Prado-Wohlwend S, de Trabajo de Endocrinología de la SEMNIM G. Functional imaging of adrenal cortex[J]. Rev Esp Med Nucl Imagen Mol(Engl Ed), 2020, 39(6): 393-404.
[9] Lu CC, Yen RF, Peng KY, et al. NP-59 adrenal scintigraphy as an imaging biomarker to predict KCNJ5 mutation in primary aldosteronism patients[J]. Front Endocrinol, 2021, 12: 644927. doi: 10.3389/fendo.2021.644927
[10] Wu MH, Liu FH, Lin KJ, et al. Diagnostic value of adrenal iodine-131 6-beta-iodomethyl-19-norcholesterol scintigraphy for primary aldosteronism: a retrospective study at a medical center in north Taiwan[J]. Nucl Med Commun, 2019, 40(6): 568-575. doi: 10.1097/MNM.0000000000000987
[11] Ren XY, Cheng G, Wang ZJ. Advances in the molecular imaging of primary aldosteronism[J]. Ann Nucl Med, 2023, 37(8): 433-441. doi: 10.1007/s12149-023-01851-y
[12] Brooks AF, Winton WP, Stauff J, et al. Development of fluorinated NP-59: a revival of cholesterol use imaging with PET[J]. J Nucl Med, 2022, 63(12): 1949-1955. doi: 10.2967/jnumed.122.263864
[13] Wu XL, Senanayake R, Goodchild E, et al. [11C]metomidate PET-CT versus adrenal vein sampling for diagnosing surgically curable primary aldosteronism: a prospective, within-patient trial[J]. Nat Med, 2023, 29(1): 190-202. doi: 10.1038/s41591-022-02114-5
[14] Isojärvi J, Viukari M, Pörsti I, et al. Lateralization in 11C-Metomidate PET and outcome of adrenalectomy in primary aldosteronism[J]. Endocrinol Diabetes Metab, 2022, 5(6): e368. doi: 10.1002/edm2.368
[15] Mitterhauser M, Wadsak W, Wabnegger L, et al. In vivo and in vitro evaluation of [18F]FETO with respect to the adrenocortical and GABAergic system in rats[J]. Eur J Nucl Med Mol Imaging, 2003, 30(10): 1398-1401. doi: 10.1007/s00259-003-1252-8
[16] Chen Cardenas SM, Santhanam P. 11C-metomidate PET in the diagnosis of adrenal masses and primary aldosteronism: a review of the literature[J]. Endocrine, 2020, 70(3): 479-487. doi: 10.1007/s12020-020-02474-3
[17] Hennings J, Lindhe O, Bergström M, et al. 11C-metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings[J]. J Clin Endocrinol Metab, 2006, 91(4): 1410-1414. doi: 10.1210/jc.2005-2273
[18] Soinio M, Luukkonen AK, Seppänen M, et al. Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism[J]. Eur J Endocrinol, 2020, 183(6): 539-550. doi: 10.1530/EJE-20-0532
[19] O'Shea PM, O'Donoghue D, Bashari W, et al. 11C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice[J]. Clin Endocrinol, 2019, 90(5): 670-679. doi: 10.1111/cen.13942
[20] Lu CC, Chen CJ, Peng KY, et al. Predicting treatment response in primary aldosteronism using 11C-metomidate positron emission tomography[J]. Clin Nucl Med, 2022, 47(11): 936-942. doi: 10.1097/RLU.0000000000004369
[21] Takeda Y, Demura M, Kometani M, et al. Molecular and epigenetic control of aldosterone synthase, CYP11B2 and 11-hydroxylase, CYP11B1[J]. Int J Mol Sci, 2023, 24(6): 5782. doi: 10.3390/ijms24065782
[22] Puar TH, Khoo CM, Tan CJ, et al. 11C-metomidate PET-CT versus adrenal vein sampling to subtype primary aldosteronism: a prospective clinical trial[J]. J Hypertens, 2022, 40(6): 1179-1188. doi: 10.1097/HJH.0000000000003132
[23] Hahner S, Stuermer A, Kreissl M, et al. [123I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes[J]. J Clin Endocrinol Metab, 2008, 93(6): 2358-2365. doi: 10.1210/jc.2008-0050
[24] Hahner S, Kreissl MC, Fassnacht M, et al. Functional characterization of adrenal lesions using[123I]IMTO-SPECT/CT[J]. J Clin Endocrinol Metab, 2013, 98(4): 1508-1518. doi: 10.1210/jc.2012-3045
[25] Heinze B, Schirbel A, Nannen L, et al. Novel CYP11B-ligand [123/131I]IMAZA as promising theranostic tool for adrenocortical tumors: comprehensive preclinical characterization and first clinical experience[J]. Eur J Nucl Med Mol Imaging, 2021, 49(1): 301-310. doi: 10.1007/s00259-021-05477-y
[26] Lindenberg L, Ahlman M, Lin F, et al. Advances in PET imaging of the CXCR4 receptor: [68Ga]Ga-PentixaFor[J]. Semin Nucl Med, 2024, 54(1): 163-170. doi: 10.1053/j.semnuclmed.2023.09.002
[27] Kawaguchi N, Zhang TT, Nakanishi T. Involvement of CXCR4 in normal and abnormal development[J]. Cells, 2019, 8(2): 185. doi: 10.3390/cells8020185
[28] Heinze B, Fuss CT, Mulatero P, et al. Targeting CXCR4(CXC chemokine receptor type 4) for molecular imaging of aldosterone-producing adenoma[J]. Hypertension, 2018, 71(2): 317-325. doi: 10.1161/HYPERTENSIONAHA.117.09975
[29] Ding J, Zhang YS, Wen J, et al. Imaging CXCR4 expression in patients with suspected primary hyperaldosteronism[J]. Eur J Nucl Med Mol Imaging, 2020, 47(11): 2656-2665. doi: 10.1007/s00259-020-04722-0
[30] Ding J, Tong AL, Zhang YS, et al. Intense 68Ga-pentixafor activity in aldosterone-producing adrenal adenomas[J]. Clin Nucl Med, 2020, 45(4): 336-339. doi: 10.1097/RLU.0000000000002946
[31] Hu JB, Xu TT, Shen H, et al. Accuracy of Gallium-68 pentixafor positron emission tomography-computed tomography for subtyping diagnosis of primary aldosteronism[J]. JAMA Netw Open, 2023, 6(2): e2255609. doi: 10.1001/jamanetworkopen.2022.55609
[32] Chaman Baz AH, van de Wiel E, Groenewoud H, et al. CXCR4-directed[68Ga]Ga-PentixaFor PET/CT versus adrenal vein sampling performance: a study protocol for a randomised two-step controlled diagnoStic trial ultimately comparing hypertenSion outcome in primary aldosteronism(CASTUS)[J]. BMJ Open, 2022, 12(8): e060779. doi: 10.1136/bmjopen-2022-060779
[33] Kološová B, Waldauf P, Wichterle D, et al. Validation of existing clinical prediction tools for primary aldosteronism subtyping[J]. Diagnostics, 2022, 12(11): 2806. doi: 10.3390/diagnostics12112806
[34] Burrello J, Burrello A, Pieroni J, et al. Development and Validation of Prediction Models for Subtype Diagnosis of Patients With Primary Aldosteronism[J]. J Clin Endocrinol Metab, 2020, 105(10): dgaa379.
[35] Shi SM, Tian Y, Ren Y, et al. A new machine learning-based prediction model for subtype diagnosis in primary aldosteronism[J]. Front Endocrinol, 2022, 13: 1005934. doi: 10.3389/fendo.2022.1005934
-

计量
- 文章访问数: 61
- 施引文献: 0



 下载:
下载: