Metabolic phenotype of enzalutamide-resistant castration-resistant prostate cancer cells revealed by untargeted metabolomics
-
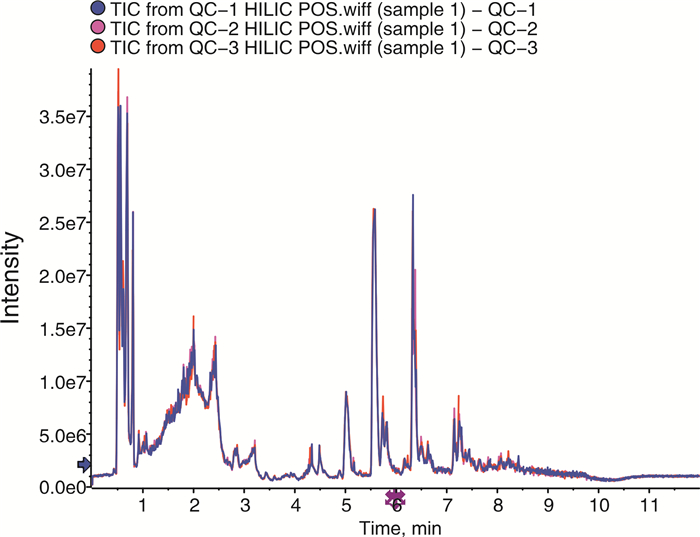
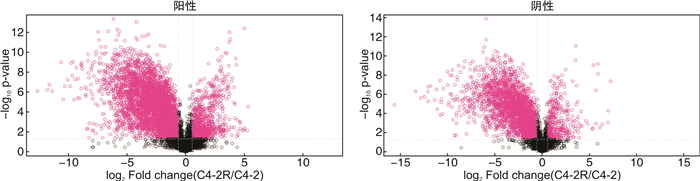
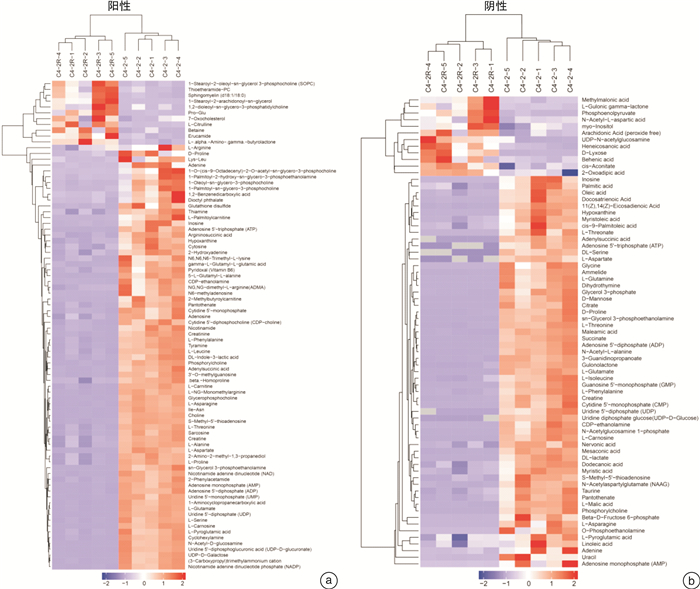
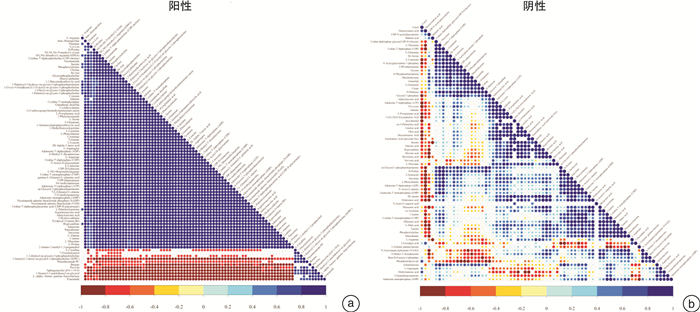
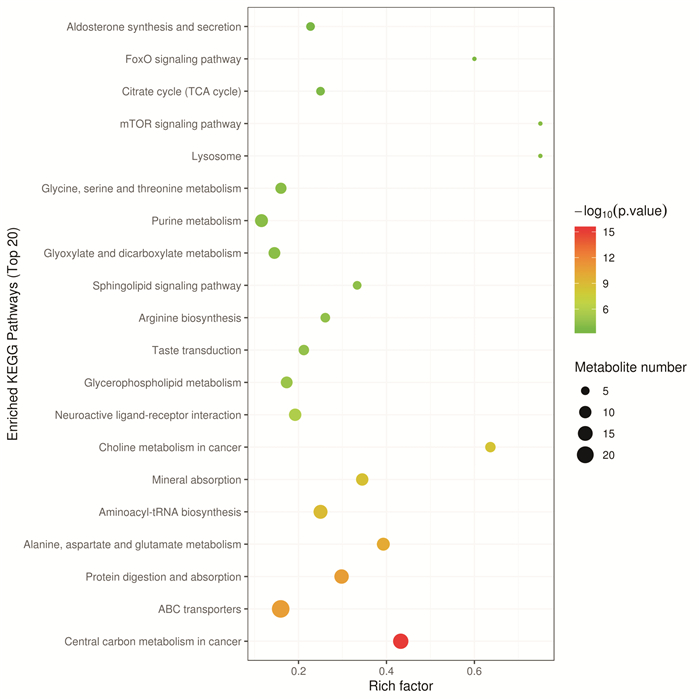
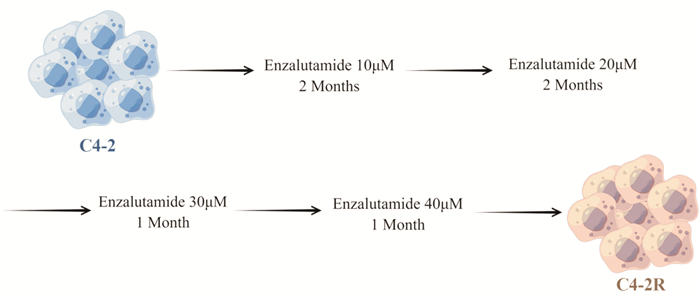
摘要: 目的 采用超高效液相色谱-四级杆串联飞行时间质谱(UHPLC-Q-TOF/MS)法分析恩杂鲁胺耐药的去势抵抗性前列腺癌细胞(CRPC)原型成分及代谢产物,探讨恩杂鲁胺耐药CRPC所改变的代谢物和途径,为临床诊断和治疗提供帮助。方法 以液相色谱-质谱法(LC-MS)检测其CRPC细胞系C4-2和恩杂鲁胺耐药CRPC细胞系C4-2R,通过单因素分析和多因素(主成分分析及偏最小二乘回归分析法等)鉴定组间正、负离子模式下代谢谱差异,通过代谢物通路的关键富集分析揭示可能介导恩杂鲁胺耐药的异常代谢途径。结果 质量控制实验表明数据稳定可靠,代谢谱差异能显著反映组间样本的代谢组学差异。单因素和多因素结果表明显示出两组样本间代谢物具有显著差异,共分别鉴定出正离子和负离子模式下显著差异代谢物83个和71个,功能富集结果表明癌症多种生物合成等重要通路发生了显著变化,体现为过度消耗状态,并以癌症中的胆碱代谢最为显著,说明CRPC细胞中恩杂鲁胺耐药过程的胆碱代谢发生了显著上调,改变胆碱代谢途径可能为逆转CRPC恩杂鲁胺耐药提供更多证据。结论 恩杂鲁胺耐药使CRPC细胞的代谢谱发生显著改变,主要表现在能量代谢上的过度消耗,表现为以胆碱能代谢升高为主的过度消耗状态,从而为晚期前列腺癌的精准化、个性化诊治和长期药物管理提供新的方向。Abstract: Objective To analyze the prototype components and metabolites of enzalutamide-resistant castration-resistant prostate cancer(CRPC) cells by ultra-high performance liquid chromatography and quadrupole tandem time-of-flight mass spectrometry(UHPLC-Q-TOF/MS), and explore the metabolites and pathways altered by Enzalutamide-resistant CRPC for clinical diagnosis and treatment.Methods The CRPC cell line C4-2 and the Enzalutamide-resistant CRPC cell line C4-2R were detected by liquid chromatography-mass spectrometry(LC-MS). Univariate and multivariate analyses(including principal component analysis and partial least squares regression analysis) were used to identify differences in metabolic profiles between groups in positive and negative ion modes. The functional enrichment analysis of metabolite pathways revealed the abnormal metabolic pathways mediated by resistance of Enzalutamide for CRPC.Results The quality control experiments showed the stable data and reliable metabolomic differences between the groups. Univariate and multivariate results showed that there were significant differences in metabolites between the C4-2 and C4-2R cells. A total of 83 and 71 metabolites with significant differences in positive and negative ion modes were identified, respectively. The results of functional enrichment showed that the important pathways of various biosynthesis in cancers have undergone significant changes, which were reflected in the state of over-consumption, and choline metabolism in CRPC is the most significant. These findings indicated that the choline metabolism was significantly up-regulated during the Enzalutamide-resistance process in CRPC cells, and alteration of the choline metabolic pathway may provide more evidence for reversing the Enzalutamide-resistance in CRPC cells.Conclusion Enzalutamide resistance significantly changes the metabolic profile of CRPC cells, which is mainly manifested in the excessive consumption of energy metabolism. Enzalutamide-resistant CRPC cells were mainly manifested as an excessive consumption state with low cholinergic metabolism, which shed novel lights on the personalized diagnosis and treatment and long-term drug management of CRPC.
-

-
[1] Zheng R. Cancer incidence and mortality in China, 2016[J]. J Nat Cancer Cent, 2022, 10: 200.
[2] Xu W, Anwaier A, Ma C, et al. Prognostic immunophenotyping clusters of clear cell renal cell carcinoma defined by the unique tumor immune microenvironment[J]. Front Cell Dev Biol, 2021, 9: 785410. doi: 10.3389/fcell.2021.785410
[3] Tayanloo-Beik A, Sarvari M, Payab M, et al. OMICS insights into cancer histology; Metabolomics and proteomics approach[J]. Clin Biochem, 2020, 84: 13-20. doi: 10.1016/j.clinbiochem.2020.06.008
[4] Li X, Gulati M, Larson AC, et al. Immune checkpoint blockade in pancreatic cancer: Trudging through the immune desert[J]. Semin Cancer Biol, 2022, 86(Pt 2): 14-27.
[5] Schmidt DR, Patel R, Kirsch DG, et al. Metabolomics in cancer research and emerging applications in clinical oncology[J]. CA Cancer J Clin, 2021, 71(4): 333-358. doi: 10.3322/caac.21670
[6] Pan J. Stereotactic Radiotherapy for Lesions Detected via(68) Ga-Prostate-specific Membrane Antigen and(18) F-Fluorodexyglucose Positron Emission Tomography/Computed Tomography in Patients with Nonmetastatic Prostate Cancer with Early Prostate-specific Antigen Progression on Androgen Deprivation Therapy: A Prospective Single-center Study[J]. Eur Urol Oncol, 2022.5(4): 420-427. doi: 10.1016/j.euo.2022.02.002
[7] Wang B. A Prospective Trial of(68) Ga-PSMA and(18) F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients with an Early PSA Progression During Castration[J]. Clin Cancer Res, 2020, 26(17): 4551-4558. doi: 10.1158/1078-0432.CCR-20-0587
[8] Verma S, Shankar E, Chan ER, et al. Metabolic reprogramming and predominance of solute carrier genes during acquired enzalutamide resistance in prostate cancer[J]. Cells, 2020, 9(12): 111.
[9] Yu L, Lai Q, Feng Q, et al. Serum metabolic profiling analysis of chronic gastritis and gastric cancer by untargeted metabolomics[J]. Front Oncol, 2021, 11: 636917. doi: 10.3389/fonc.2021.636917
[10] Zhu Y. Epidemiology and genomics of prostate cancer in Asian men[J]. Nat Rev Urol, 2021, 18(5): 282-301. doi: 10.1038/s41585-021-00442-8
[11] Trogdon JG, Falchook AD, Basak R, et al. Total medicare costs associated with diagnosis and treatment of prostate cancer in elderly men[J]. JAMA Oncol, 2019, 5(1): 60-66. doi: 10.1001/jamaoncol.2018.3701
[12] Smith MR. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer[J]. N Engl J Med, 2022, 386(12): 1132-1142. doi: 10.1056/NEJMoa2119115
[13] Zhu Y, Ye D. Chinese expert consensus on the diagnosis and treatment of castration-resistant prostate cancer(2019 update)[J]. Cancer Manag Res, 2020, 12: 2127-2140. doi: 10.2147/CMAR.S236879
[14] Sweeney CJ, Martin AJ, Stockler MR, et al. Overall survival of men with metachronous metastatic hormone-sensitive prostate cancer treated with enzalutamide and androgen deprivation therapy[J]. Eur Urol, 2021, 80(3): 275-279. doi: 10.1016/j.eururo.2021.05.016
[15] Attard G. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol[J]. Lancet, 2022, 399(10323): 447-460. doi: 10.1016/S0140-6736(21)02437-5
[16] van Soest RJ, van Royen ME, de Morrée ES, et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer[J]. Eur J Cancer, 2013, 49(18): 3821-3830. doi: 10.1016/j.ejca.2013.09.026
[17] Gao L. KIF15-Mediated Stabilization of AR and AR-V7 Contributes to Enzalutamide Resistance in Prostate Cancer[J]. Cancer Res, 2021, 81(4): 1026-1039. doi: 10.1158/0008-5472.CAN-20-1965
[18] Wang J, Xu W, Wang B, et al. GLUT1 is an AR target contributing to tumor growth and glycolysis in castration-resistant and enzalutamide-resistant prostate cancers[J]. Cancer Lett, 2020, 485: 45-55. doi: 10.1016/j.canlet.2020.05.007
[19] 赫捷. 中国前列腺癌筛查与早诊早治指南(2022, 北京)[J]. 中华肿瘤杂志, 2022, 44(1): 29-53. https://www.cnki.com.cn/Article/CJFDTOTAL-ZHLU202201001.htm
[20] Siegel RL. Cancer Statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. doi: 10.3322/caac.21654
[21] Gillessen S. Management of Patients with Advanced Prostate Cancer: Report from the Advanced Prostate Cancer Consensus Conference 2021[J]. Eur Urol, 2022, 82(1): 115-141. doi: 10.1016/j.eururo.2022.04.002
[22] 丁银芳, 田伟. 前列腺癌中雄激素受体信号通路与其他信号通路的相互作用[J]. 临床泌尿外科杂志, 2022, 37(4): 319-325. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMW202204016.htm
[23] 冯涛. 前列腺癌恩杂鲁胺耐药细胞模型的构建及潜在耐药基因的初步筛选和验证[J]. 癌症, 2021, 40(11): 486-498. doi: 10.3321/j.issn.1000-467X.2021.11.ez202111003
[24] 高杰. 脂质代谢在去势抵抗性前列腺癌中的研究进展[J]. 东南国防医药, 2021, 23(2): 169-174. doi: 10.3969/j.issn.1672-271X.2021.02.013
[25] Sun BL. Immunotherapy in treatment of metastatic prostate cancer: An approach to circumvent immunosuppressive tumor microenvironment[J]. Prostate, 2021, 81(15): 1125-1134. doi: 10.1002/pros.24213
[26] Matos A. Arginine and Arginases Modulate Metabolism, Tumor Microenvironment and Prostate Cancer Progression[J]. Nutrients, 2021, 13(12): 211.
[27] Kouspou MM, Fong JE, Brew N, et al. The movember prostate cancer landscape analysis: an assessment of unmet research needs[J]. Nat Rev Urol, 2020, 17(9): 499-512. doi: 10.1038/s41585-020-0349-1
[28] 瞿根义. 前列腺癌对多西他赛耐药相关基因的生物信息学分析[J]. 临床泌尿外科杂志, 2020, 35(7): 510-515. doi: 10.13201/j.issn.1001-1420.2020.07.002
[29] 徐文浩. 人工智能在泌尿系统肿瘤中的应用研究进展[J]. 中国癌症杂志, 2022, 32(1): 68-74. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGAZ202201010.htm
[30] Fan Z. Crosstalk of FGFR1 signaling and choline metabolism promotes cell proliferation and survival in prostate cancer cells[J]. Int J Cancer, 2022, 150(9): 1525-1536. doi: 10.1002/ijc.33922
[31] Beier AK, Puhr M, Stope MB, et al. Metabolic changes during prostate cancer development and progression[J]. J Cancer Res Clin Oncol, 2022, Sep 23. doi: 10.1007/s00432-022-04371-w.
[32] Saiki I, Yara M, Yamanaka T, et al. Functional Expression of Choline Transporter-Like Protein 1 in LNCaP Prostate Cancer Cells: A Novel Molecular Target[J]. Biomol Ther(Seoul), 2020, 28(2): 195-201. doi: 10.4062/biomolther.2019.097
[33] Giovacchini G, Giovannini E, Borsò E, et al. Sensitivity of fluorine-18-fluoromethylcholine PET/CT to prostate-specific antigen over different plasma levels: a retrospective study in a cohort of 192 patients with prostate cancer[J]. Nucl Med Commun, 2019, 40(3): 258-263. doi: 10.1097/MNM.0000000000000959
[34] Garg I. (11) C-choline positron emission tomography/computed tomography for detection of disease relapse in patients with history of biochemically recurrent prostate cancer and prostate-specific antigen < /=0.1 ng/ml[J]. J Cancer Res Ther, 2021, 17(2): 358-365. doi: 10.4103/jcrt.JCRT_373_19
[35] García JR, Compte A, Buxeda M, et al. Value of 18F-Choline PET/MRI hybrid technique on the therapeutic approach for patients with prostate cancer treated with prostatectomy and rising prostate specific antigen levels below 1 ng/ml[J]. Rev Esp Med Nucl Imagen Mol(Engl Ed), 2020, 39(4): 197-203.
[36] Michaud L. (11) C-Choline PET/CT in Recurrent Prostate Cancer: Retrospective Analysis in a Large U.S. Patient Series[J]. J Nucl Med, 2020, 61(6): 827-833.
[37] Urso L. 18F-Choline PET/CT or PET/MR and the evaluation of response to systemic therapy in prostate cancer: are we ready?[J]. Clin Transl Imaging, 2022, 30: 1-9.
[38] Telo S. Negative 11C-choline PET/computed tomography imaging in restaging of patients with prostate cancer with serum prostate-specific antigen values>20 ng/mL[J]. Nucl Med Commun, 2020, 41(11): 1178-1182.
[39] Wen S. Aberrant activation of super enhancer and choline metabolism drive antiandrogen therapy resistance in prostate cancer[J]. Oncogene, 2020, 39(42): 6556-6571.
[40] Kimura K, Kitajima K, Kawanaka Y, et al. Evaluation of 11 C-choline positron emission tomography/computed tomography for determining treatment response in castration-resistant prostate cancer patients[J]. Int J Urol, 2022, 29(9): 1072-1078.
[41] Caroli P, De Giorgi U, Scarpi E, et al. Prognostic value of 18F-choline PET/CT metabolic parameters in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide[J]. Eur J Nucl Med Mol Imaging, 2018, 45(3): 348-354.
-




 下载:
下载: