-
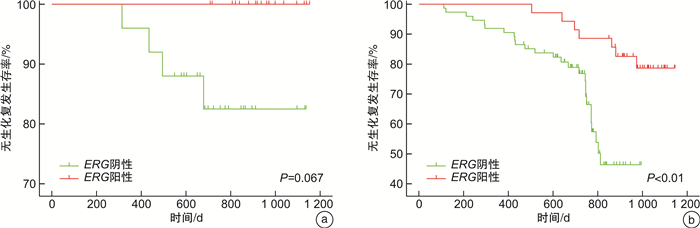
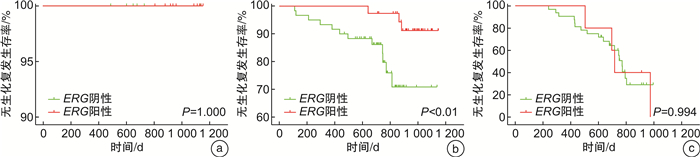
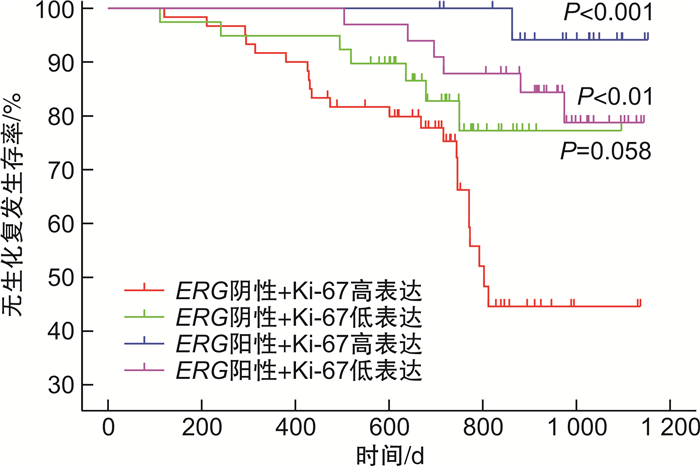
摘要: 目的 通过前瞻性队列研究,探讨ETS相关基因(ETS-related gene,ERG)扩增水平作为前列腺癌根治术患者预后预测指标的价值。方法 通过荧光原位杂交的方法检测前列腺癌根治术患者组织样本中的ERG扩增水平,与患者的血清PSA水平、病理Gleason评分、病理分期、Ki-67表达水平进行相关性分析,并对患者的生化复发情况进行预测。结果 前瞻性队列共纳入152例接受前列腺癌根治术的患者,其中有53例的组织标本为ERG阳性(34.9%)。ERG扩增水平与病理Gleason评分(P < 0.05)和Ki-67表达水平(P < 0.05)显著相关。ERG阳性率在Gleason评分6分、7分和>7分组中分别为59%、39%和14%,而在Ki-67阳性比例 < 2%、2%~10%和>10%组中分别为46%、26%和22%。此外,ERG扩增阳性在低血清PSA(P=0.256)和低病理T分期(P=0.200)的患者中更常见,但差异无统计学意义。Cox回归分析发现ERG阴性(HR=0.42,95%CI:0.17~1.03)和Ki-67高表达(HR=1.00,95%CI:0.96~1.02)均是前列腺癌根治术后患者生化复发的独立危险因素。根据Kaplan-Meier生存分析证实ERG阴性同时Ki-67高表达组的患者无生化复发生存时间最短,其次是ERG阴性同时Ki-67低表达组(P=0.058)和ERG阳性同时Ki-67低表达组(P < 0.01),而ERG阳性同时Ki-67高表达组(P < 0.001)的无疾病进展生存时间最长。结论 在ERG整体阳性比例较低的人群中,ERG扩增水平是前列腺癌根治术后患者生化复发的独立预后因素。ERG与Ki-67等其他生物标志物的联合应用具有更理想的预测效能。Abstract: Objective To prospectively investigate the value of ETS-related gene(ERG) as a prognostic factor in patients undergoing radical prostatectomy.Methods The amplification level of ERG in tissue samples of patients undergoing radical prostatectomy was detected by fluorescence in situ hybridization. The correlation between ERG amplification level and serum PSA level, Gleason score, pathological stage and Ki-67 expression level were analyzed, and the predictive value for biochemical recurrence was estimated.Results A total of 152 patients who underwent radical prostatectomy were included, of which ERG was positive in 53 prostate cancer samples(34.9%). ERG was significantly associated with postoperative Gleason score(P < 0.05) and Ki-67 positive percentage(P < 0.05). The positive rate of ERG was significantly higher in patients with Gleason score=6(59%), compared with those having Gleason score=7(39%) or Gleason score>7(14%). The positive rate of ERG was significantly higher in patients with Ki-67 positive percentage < 2%(46%), compared with those having Ki-67 positive percentage 2%-10%(26%) and > 10%(22%). Furthermore, positive expression of ERG occurred more frequently in patients with lower PSA(P=0.256) and lower pathological T stage(P=0.200), but without statistically significant. Cox regression analysis showed that both ERG negative(HR=0.42, 95%CI: 0.17-1.03) and high Ki-67 expression(HR=1.00, 95%CI: 0.96-1.02) were independent risk factors for biochemical recurrence in patients after radical prostatectomy. Kaplan-Meier survival analysis confirmed that the subset of patients with negative ERG and high Ki-67 had the significant shorter biochemical recurrence-free survival compared to those with positive ERG and high Ki-67(P < 0.001) or those with positive ERG and low Ki-67(P < 0.01), while marginally shorter survival compared to those with negative ERG and low Ki-67(P=0.058).Conclusion Among the population with a low prevalence of ERG positive, ERG status is an independent prognostic factor for survival outcomes in patients undergoing radical prostatectomy. The combined application of ERG with other biomarkers such as Ki-67 might have better potential predictive value.
-
Key words:
- prostate cancer /
- radical prostatectomy /
- ETS-related gene /
- biomarker /
- survival prognosis
-

-
表 1 亚组分析中ERG扩增阳性患者所占比例的比较
例 临床病理特征 总病例数(152例) 病例数占比/% ERG扩增 阴性(99例) 阳性(53例) 阳性占比/% P值 PSA水平/(ng/mL) 0.26 < 10 43 28 25 18 42 ≥10 109 72 74 35 32 Gleason评分 6 17 11 7 10 59 < 0.05 7 98 65 60 38 39 > 7 37 24 32 5 14 病理T分期 0.20 T2a~b 24 16 13 11 46 T2c 76 50 47 29 38 T3a 30 20 21 9 30 T3b 22 14 18 4 18 Ki-67阳性比例/% < 0.05 < 2 72 47 39 33 46 2~10 53 35 39 14 26 >10 27 18 21 6 22 表 2 影响前列腺癌根治术后生化复发的危险因素Cox回归分析
临床病理特征 单因素Cox回归 多因素Cox回归 HR 95% CI P值 HR 95% CI P值 ERG扩增水平 0.22 0.09~0.52 < 0.001 0.42 0.17~1.03 < 0.05 年龄 1.00 0.96~1.04 0.913 PSA水平 3.62 1.29~10.16 < 0.010 Gleason评分 3.23 2.10~4.98 < 0.001 2.81 1.79~4.43 < 0.01 病理T分期 1.87 1.33~2.61 < 0.001 Ki-67蛋白水平 1.53 1.03~2.27 < 0.050 1.00 0.96~1.02 < 0.05 -
[1] 韩苏军, 刘飞, 邢念增. 1988—2015年中国肿瘤登记地区前列腺癌发病趋势分析[J]. 中华泌尿外科杂志, 2022, 43(1): 51-55.
[2] Clark JP, Cooper CS. ETS gene fusions in prostate cancer[J]. Nat Rev Urol, 2009, 6(8): 429-439. doi: 10.1038/nrurol.2009.127
[3] Spencer ES, Johnston RB, Gordon RR, et al. Prognostic value of ERG oncoprotein in prostate cancer recurrence and cause-specific mortality[J]. Prostate, 2013, 73(9): 905-912. doi: 10.1002/pros.22636
[4] Ren SC, Wei GH, Liu DB, et al. Whole-genome and transcriptome sequencing of prostate cancer identify new genetic alterations driving disease progression[J]. Eur Urol, 2018, 73(3): 322-339. doi: 10.1016/j.eururo.2017.08.027
[5] 周文浩, 龚志勇, 廖波, 等. ERG蛋白表达与前列腺癌患者根治术后生化复发风险关系的研究[J]. 国际泌尿系统杂志, 2019, 39(1): 1-4. doi: 10.3760/cma.j.issn.1673-4416.2019.01.001
[6] Hermans KG, van Marion R, van Dekken H, et al. TMPRSS2: ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer[J]. Cancer Res, 2006, 66(22): 10658-10663. doi: 10.1158/0008-5472.CAN-06-1871
[7] Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer[J]. Nat Rev Cancer, 2008, 8(7): 497-511. doi: 10.1038/nrc2402
[8] Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer[J]. Oncogene, 2008, 27(3): 253-263. doi: 10.1038/sj.onc.1210640
[9] Minner S, Enodien M, Sirma H, et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy[J]. Clin Cancer Res, 2011, 17(18): 5878-5888. doi: 10.1158/1078-0432.CCR-11-1251
[10] Kron K, Liu LY, Trudel D, et al. Correlation of ERG expression and DNA methylation biomarkers with adverse clinicopathologic features of prostate cancer[J]. Clin Cancer Res, 2012, 18(10): 2896-2904. doi: 10.1158/1078-0432.CCR-11-2901
[11] Tomlins SA, Alshalalfa M, Davicioni E, et al. Characterization of 1577 primary prostate cancers reveals novel biological and clinicopathologic insights into molecular subtypes[J]. Eur Urol, 2015, 68(4): 555-567. doi: 10.1016/j.eururo.2015.04.033
[12] Couñago F, López-Campos F, Díaz-Gavela AA, et al. Clinical applications of molecular biomarkers in prostate cancer[J]. Cancers, 2020, 12(6): 1550. doi: 10.3390/cancers12061550
[13] Wasim S, Lee SY, Kim J. Complexities of prostate cancer[J]. Int J Mol Sci, 2022, 23(22): 14257. doi: 10.3390/ijms232214257
[14] Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer[J]. Science, 2005, 310(5748): 644-648. doi: 10.1126/science.1117679
[15] Furusato B, Tan SH, Young D, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification[J]. Prostate Cancer Prostatic Dis, 2010, 13(3): 228-237. doi: 10.1038/pcan.2010.23
[16] Chaux A, Albadine R, Toubaji A, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas[J]. Am J Surg Pathol, 2011, 35(7): 1014-1020. doi: 10.1097/PAS.0b013e31821e8761
[17] Braun M, Goltz D, Adler D, et al. ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer: a comparative study of two monoclonal antibodies[J]. Prostate Cancer Prostatic Dis, 2012, 15(2): 165-169. doi: 10.1038/pcan.2011.67
[18] Morais CE, Gurgel DC, Teixeira AC, et al. Prevalence of ERG expression and PTEN loss in a Brazilian prostate cancer cohort[J]. Rev Bras De Pesquisas Med E Biol, 2019, 52(12): e8483.
[19] Abdelsalam RA, Khalifeh I, Box A, et al. Molecular characterization of prostate cancer in Middle Eastern population highlights differences with Western populations with prognostic implication[J]. J Cancer Res Clin Oncol, 2020, 146(7): 1701-1709. doi: 10.1007/s00432-020-03221-x
[20] Zhou CK, Young D, Yeboah ED, et al. TMPRSS2: ERG gene fusions in prostate cancer of West African men and a meta-analysis of racial differences[J]. Am J Epidemiol, 2017, 186(12): 1352-1361. doi: 10.1093/aje/kwx235
[21] Lee K, Chae JY, Kwak C, et al. TMPRSS2-ERG gene fusion and clinicopathologic characteristics of Korean prostate cancer patients[J]. Urology, 2010, 76(5): 1268. e7-1268.13. doi: 10.1016/j.urology.2010.06.010
[22] Miyagi Y, Sasaki T, Fujinami K, et al. ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples[J]. Mod Pathol, 2010, 23(11): 1492-1498. doi: 10.1038/modpathol.2010.149
[23] Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer[J]. J Clin Oncol, 2011, 29(27): 3659-3668. doi: 10.1200/JCO.2011.35.1916
[24] Magi-Galluzzi C, Tsusuki T, Elson P, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients[J]. Prostate, 2011, 71(5): 489-497. doi: 10.1002/pros.21265
[25] 董隽, 肖立, 孙忠全, 等. 前列腺癌E26转录因子家族基因融合发生率及其与临床病理指标相关性研究[J]. 中华泌尿外科杂志, 2014, 35(3): 195-199.
[26] 邹博, 龙衍, 杨智明, 等. 湖南地区前列腺癌流行病学及临床病理特征分析[J]. 中华男科学杂志, 2022, 28(9): 786-791. https://www.cnki.com.cn/Article/CJFDTOTAL-NKXB202209003.htm
[27] Lahdensuo K, Erickson A, Saarinen I, et al. Loss of PTEN expression in ERG-negative prostate cancer predicts secondary therapies and leads to shorter disease-specific survival time after radical prostatectomy[J]. Mod Pathol, 2016, 29(12): 1565-1574. doi: 10.1038/modpathol.2016.154
[28] Kovtun IV, Cheville JC, Murphy SJ, et al. Lineage relationship of Gleason patterns in Gleason score 7 prostate cancer[J]. Cancer Res, 2013, 73(11): 3275-3284. doi: 10.1158/0008-5472.CAN-12-2803
[29] Barbieri CE, Tomlins SA. Reprint of: the prostate cancer genome: perspectives and potential[J]. Urol Oncol, 2015, 33(2): 95-102. doi: 10.1016/j.urolonc.2015.01.002
[30] Berlin A, Castro-Mesta JF, Rodriguez-Romo L, et al. Prognostic role of Ki-67 score in localized prostate cancer: a systematic review and meta-analysis[J]. Urol Oncol, 2017, 35(8): 499-506. doi: 10.1016/j.urolonc.2017.05.004
[31] Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2: ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome[J]. Mod Pathol, 2008, 21(12): 1451-1460. doi: 10.1038/modpathol.2008.96
[32] Reid AH, Attard G, Ambroisine L, et al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer[J]. Br J Cancer, 2010, 102(4): 678-684. doi: 10.1038/sj.bjc.6605554
[33] Tomlins SA, Day JR, Lonigro RJ, et al. Urine TMPRSS2: ERG plus PCA3 for individualized prostate cancer risk assessment[J]. Eur Urol, 2016, 70(1): 45-53.
-

| 引用本文: | 吴涵, 庞庆阳, 花梅免, 等. ERG在前列腺癌中的表达及其与Ki-67联合的预后预测价值[J]. 临床泌尿外科杂志, 2023, 38(8): 589-595. doi: 10.13201/j.issn.1001-1420.2023.08.005 |
| Citation: | WU Han, PANG Qingyang, HUA Meimian, et al. Expression of ERG in prostate cancer and its prognostic value combined with Ki-67[J]. J Clin Urol, 2023, 38(8): 589-595. doi: 10.13201/j.issn.1001-1420.2023.08.005 |
- Figure 1.
- Figure 2.
- Figure 3.
- Figure 4.
- Figure 5.




 下载:
下载: