Effect of replacing intra-vesical chemotherapy agents on the prognosis of short-term recurring Ta and T1 stage bladder cancer
-
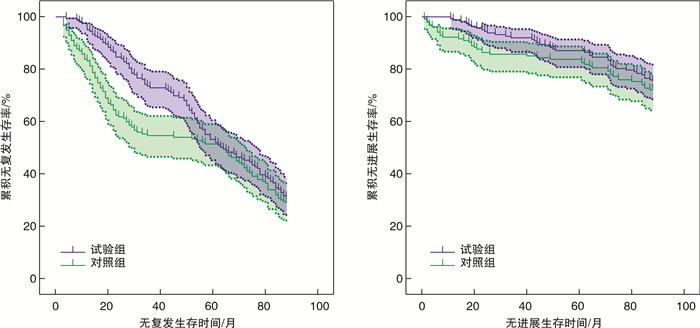
摘要: 目的 探讨更换腔内灌注化疗药物对短期复发Ta、T1期膀胱癌预后的影响。方法 回顾性分析2008年1月—2018年12月上海交通大学附属第六人民医院南院收治的316例经尿道膀胱肿瘤电切术(transurethral resection of bladder tumor,TURBT)后1年内复发的Ta、T1期膀胱癌患者的临床资料。其中162例患者再次行TURBT后更换腔内灌注化疗药物(试验组)。其他154例患者TURBT术后仍继续应用原来的腔内灌注化疗药物(对照组)。所有患者TURBT术后均即刻灌注腔内化疗药物表柔比星(epirubicin,EPI)、吡柔比星(pirarubicin,THP)或羟基喜树碱(hydroxycamptothecin,HCPT),并随后按疗程进行持续灌注。观察2组患者肿瘤复发及进展情况。结果 2组患者(试验组vs对照组)1、3、5年无复发生存率(recurrence-free survival,RFS)分别为95.06% vs 83.12%(χ2=11.73,P < 0.01)、72.84% vs 54.55%(χ2=11.46,P < 0.01)、53.08% vs 51.30%(χ2=0.10,P=0.82);无进展生存率(progression-free survival,PFS)分别为98.77% vs 92.21%(χ2=8.02,P=0.01)、91.98% vs 85.71%(χ2=3.14,P=0.11)、87.04% vs 83.77%(χ2=0.68,P=0.43)。试验组患者的1、3年RFS及1年PFS明显高于对照组,而2组间5年RFS及3、5年PFS比较差异均无统计学意义。结论 TURBT术后更换腔内灌注化疗药物可以显著降低短期复发NMIBC患者的早期复发率,但对于远期预后差异无统计学意义。Abstract: Objective To evaluate the effect of replacing intra-vesical chemotherapy agents on the prognosis of short-term recurring Ta and T1 stage bladder cancer.Methods We retrospectively reviewed a consecutive series of 316 patients who had been previously diagnosed with T1 or Ta urothelial bladder carcinoma and suffered short-term recurrence after initial transurethral resection of the bladder tumor(TURBT) and intra-vesical instillation between January 2008 and December 2018. After a second time TURBT, we immediately changed the intra-vesical instillation agents for 162 patients(study group), whereas the other 154 patients continued to use their initial drugs(control group). All patients received an immediate instillation of epirubicin(EPI), pirarubicin(THP) or hydroxycamptothecin(HCPT) after TURBT and followed by regular maintenance instillations. We compared the recurrence and progression rates between study and control groups.Results The 1-, 3-, and 5-year recurrence-free survival(RFS) of the two groups(study group vs control group) were 95.06% vs 83.12%(χ2=11.73, P < 0.01), 72.84% vs 54.55%(χ2=11.46, P < 0.01), 53.08% vs 51.30%(χ2=0.10, P=0.82), and progression-free survival(PFS) were 98.77% vs 92.21%(χ2=8.02, P=0.01), 91.98% vs 85.71%(χ2=3.14, P=0.11), 87.04% vs 83.77%(χ2=0.68, P=0.43), separately. The 1- and 3-year RFS and 1-year PFS of study group were higher than those of control group. However, there were no significant differences in the 5-year RFS or 3-, 5-year PFS between the two groups.Conclusion Replacement of intra-vesical chemotherapy agents for short-term recurring Ta and T1 stage bladder cancer can significantly reduce its early recurrence and progression rates, but have no significant statistical difference on long-term prognosis in this trial.
-
Key words:
- instillation therapy /
- bladder tumor /
- drug resistance /
- tumor recurrence /
- tumor progression
-

-
表 1 3种腔内化疗药物的灌注方案
腔内化疗药物 规格 单次总剂量 疗程 HCPT 20 mg 80 mg/20 mL NS 每周1次,连续8次;后改为每3周1次,连续8次;最后每个月1次,连续8次 EPI 10 mg 50 mg/50 mL GLU 每周1次,连续8次;后改为每3周1次,连续8次;最后每个月1次,连续8次 THP 10 mg 50 mg/50 mL GLU 每周1次,连续8次;后改为每3周1次,连续8次;最后每个月1次,连续8次 注:NS为生理盐水,GLU为葡萄糖。 表 2 2组患者临床特征比较
例 临床特征 试验组(162例) 对照组(154例) χ2/t P值 性别 1.59 0.22 男 122 125 女 40 29 年龄/岁 40~88 37~91 -0.87 0.39 肿瘤数量 0.03 0.91 单发 71 69 多发 91 85 肿瘤大小/cm 1.66 0.22 ≤3 107 112 > 3 55 42 病理分期 1.67 0.21 Ta 61 69 T1 101 85 肿瘤分级 1.06 0.33 高级别 117 103 低级别 45 51 表 3 2组患者1、3、5年RFS及PFS比较
例(%) 指标 试验组(162例) 对照组(154例) χ2 P值 RFS 1年 154(95.06) 128(83.12) 11.73 < 0.05 3年 118(72.84) 84(54.55) 11.46 < 0.05 5年 86(53.08) 79(51.30) 0.10 0.82 PFS 1年 160(98.77) 142(92.21) 8.02 0.01 3年 149(91.98) 132(85.71) 3.14 0.11 5年 141(87.04) 129(83.77) 0.68 0.43 -
[1] Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis[J]. Urology, 2005, 66(6 Suppl 1): 4-34.
[2] Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model[J]. J Urol, 2009, 182(5): 2195-2203. doi: 10.1016/j.juro.2009.07.016
[3] 罗松涛, 王应洪, 李绪鲲, 等. 羟喜树碱对比丝裂霉素膀胱内灌注化疗临床随机对照研究[J]. 中国临床药理学杂志, 2013, 29(6): 416-418. doi: 10.3969/j.issn.1001-6821.2013.06.005
[4] Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016[J]. Eur Urol, 2017, 71(3): 447-461. doi: 10.1016/j.eururo.2016.05.041
[5] Soloway MS, Bruck DS, Kim SS. Expectant management of small, recurrent, noninvasive papillary bladder tumors[J]. J Urol, 2003, 170(2 Pt 1): 438-441.
[6] Sobin LH, Gospodariwicz M, Wittekind C. TNM classification of malignant tumors[M]. 7th ed. New York: Wiley-Blackwell, 2009: 262-265.
[7] Lopez-Beltran A, Montironi R. Non-invasive urothelial neoplasms: according to the most recent WHO classification[J]. Eur Urol, 2004, 46(2): 170-176. doi: 10.1016/j.eururo.2004.03.017
[8] Cheng L, MacLennan GT, Lopez-Beltran A. Histologic grading of urothelial carcinoma: a reappraisal[J]. Hum Pathol, 2012, 43(12): 2097-2108. doi: 10.1016/j.humpath.2012.01.008
[9] Knowles MA. Bladder cancer subtypes defined by genomic alterations[J]. Scand J Urol Nephrol Suppl, 2008(218): 116-130.
[10] 张元芳, 张祖豹, 唐涌志, 等. 表柔比星与丝裂霉素膀胱内单次与多次灌注预防浅表性膀胱癌术后复发[J]. 中国癌症杂志, 2000, 10(3): 211-213. doi: 10.3969/j.issn.1007-3639.2000.03.006
[11] 张天禹, 张琳刚, 覃展偶, 等. MTS/PMS法在膀胱癌原代细胞化疗药物敏感性检测中的应用(附29例报告)[J]. 山东医药, 2010, 50(46): 9-10. doi: 10.3969/j.issn.1002-266X.2010.46.005
[12] 王城博, 金文军, 董治龙. 膀胱热灌注化疗的临床应用与研究进展[J]. 临床泌尿外科杂志, 2022, 37(12): 952-956. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2022.12.013
[13] 孔家瑾, 张璐, 郑克文. 膀胱癌分子机制及分子分型的研究进展[J]. 临床泌尿外科杂志, 2021, 36(3): 236-241. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2021.03.016
[14] Tada Y, Wada M, Migita T, et al. Increased expression of multidrug resistance-associated proteins in bladder cancer during clinical course and drug resistance to doxorubicin[J]. Int J Cancer, 2002, 98(4): 630-635. doi: 10.1002/ijc.10246
[15] Wang L, Huang SL, Zhang P, et al. The application of gemcitabine and pirarubicin in patients with non-muscle invasive bladder cancer[J]. J Cancer Res Clin Oncol, 2023.
[16] Daryanto B, Purnomo AF, Seputra KP, et al. Comparison between intravesical chemotherapy epirubicin and mitomycin-C after TURB vs TURB alone with recurrence rate of non-muscle invasive bladder cancer: meta-analysis[J]. Med Arch, 2022, 76(3): 198-201. doi: 10.5455/medarh.2022.76.198-201
[17] Tanimoto R, Saika T, Ebara S, et al. Prospective randomized controlled trial of postoperative early intravesical chemotherapy with pirarubicin(THP)for solitary non-muscle invasive bladder cancer comparing single and two-time instillation[J]. World J Urol, 2018, 36(6): 889-895. doi: 10.1007/s00345-018-2196-8
[18] Shao LJ, Wang HJ, Wang JR, et al. Clinical efficacy of intravesical gemcitabine combined with ubenimex in patients with non-muscle-invasive bladder carcinoma after transurethral resection of bladder tumor[J]. Pak J Med Sci, 2022, 38(5): 1243-1249.
[19] Mariappan P, Johnston A, Padovani L, et al. Enhanced quality and effectiveness of transurethral resection of bladder tumour in non-muscle-invasive bladder cancer: a multicentre real-world experience from scotland's quality performance indicators programme[J]. Eur Urol, 2020, 78(4): 520-530.
[20] 肖振东, 李长岭, 田军. 利用体外药敏试验指导膀胱癌临床个体化灌注化疗[J]. 现代泌尿生殖肿瘤杂志, 2009, 1(4): 207-209. https://www.cnki.com.cn/Article/CJFDTOTAL-PXDM200904006.htm
[21] Ramachandran C, Wellham LL. Effect of MDR1 phosphorothioate antisense oligodeoxynucleotides in multidrug-resistant human tumor cell lines and xenografts[J]. Anticancer Res, 2003, 23(3B): 2681-2690.
-




 下载:
下载: