Construction and verification of nomogram prediction model for positive risk of prostate biopsy in patients with PSA fluctuation
-
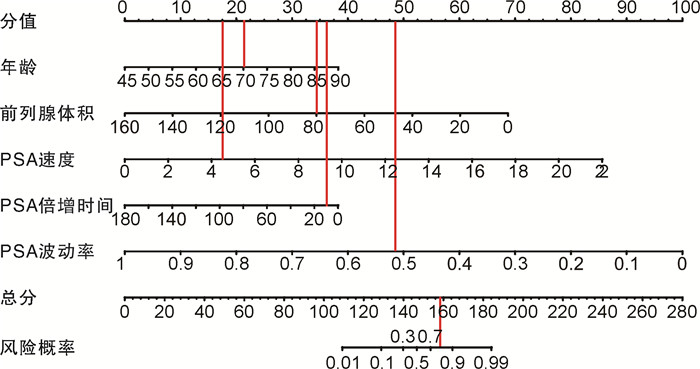
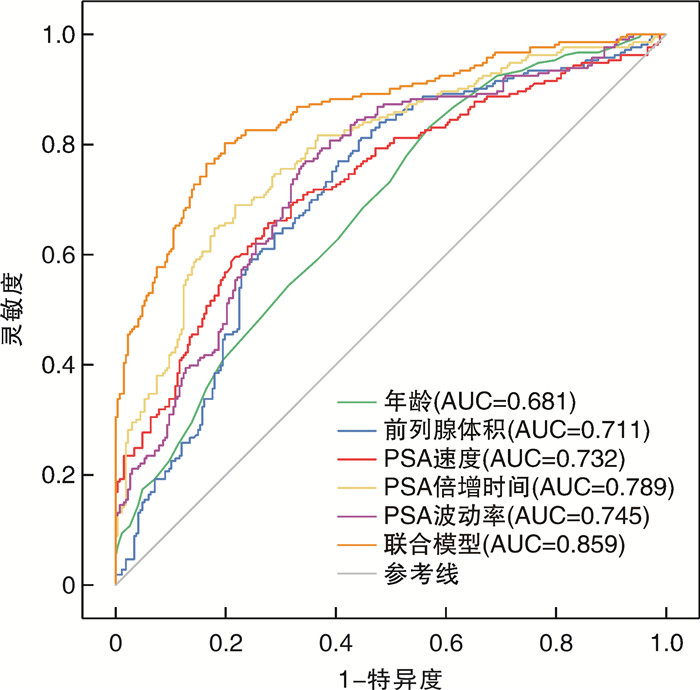
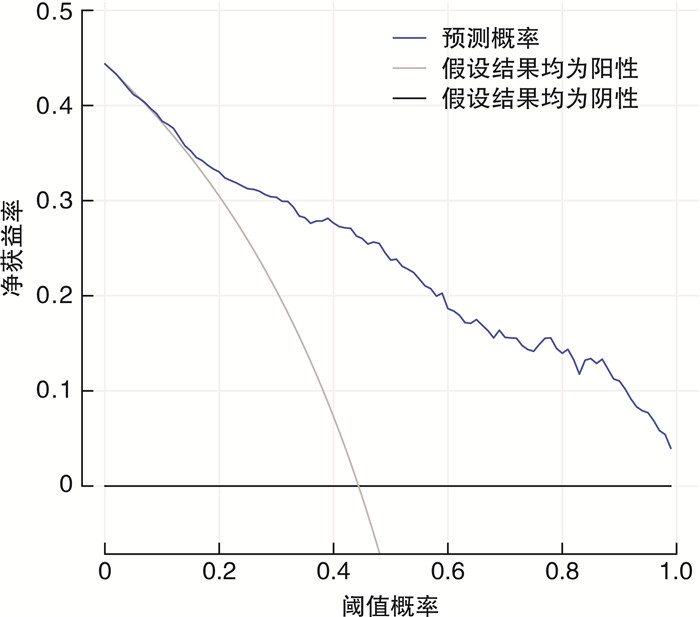
摘要: 目的 探讨≥2次行前列腺特异性抗原(prostate specific antigen,PSA)检测且每次PSA检测结果均在4~10 ng/mL的患者行前列腺穿刺活检阳性的危险因素,构建列线图预测模型并进行评估。方法 收集苏北人民医院2019年1月15日—2022年9月26日期间≥2次行PSA检测且每次PSA检测结果均在4~10 ng/mL患者的相关临床资料,穿刺方法均结合运用认知融合靶向+系统穿刺,采用单因素分析和多因素logistic回归分析筛选影响这类患者行前列腺穿刺活检阳性的危险因素,将筛选出的独立危险因素放入列线图构建预测模型,并通过受试者工作特征(receiver operating characteristic,ROC)曲线分析、校准图和决策曲线分析评估列线图模型的区分度与准确度。结果 共纳入480例患者,其中213例(44.37%)前列腺穿刺活检结果为阳性,多因素logistic回归分析显示,年龄、前列腺体积、PSA速度、PSA倍增时间、PSA波动率是该类患者行前列腺穿刺活检阳性的危险因素(P < 0.05);基于多因素logistic回归分析构建列线图预测模型,ROC曲线下面积为0.859,内部验证校准良好,列线图校准曲线斜率接近1,Hosmer-Lemeshow拟合优度检验为20.869,P=0.171。结论 基于本次研究构建的列线图模型,具有良好的区分度与一致性,可以为≥2次行PSA检测且每次PSA检测结果均在4~10 ng/mL的患者了解患病风险及泌尿科医生做出临床决策提供有效帮助。
-
关键词:
- 前列腺肿瘤 /
- 前列腺穿刺 /
- 前列腺特异性抗原动力学 /
- 预测模型
Abstract: Objective To explore the risk factors of positive prostate biopsy in patients with two or more prostate specific antigen(PSA) tests and each PSA test result in 4-10 ng/mL, and to construct a nomogram prediction model and evaluate it.Methods We collected clinical data related to the population of patients who underwent two or more PSA tests at Subei People's Hospital from January 15th, 2019 to September 26th, 2022, with each PSA test result ranging from 4 to 10 ng/mL. Univariate analysis and multivariate logistic regression analysis were used to screen for risk factors that affected two or more PSA tests and each PSA test result ranging from 4 to 10 ng/mL, then the screened independent risk factors were put into the nomogram to build a prediction model. The differentiation and accuracy of the nomogram model were evaluated through receiver operating characteristic (ROC) analysis, calibration chart and decision curve analysis.Results A total of 480 patients with two or more PSA tests and each PSA test 4-10 ng/mL were included in this retrospective study. Among them, 213 (44.37%) prostate biopsy results were positive. Multivariate logistic regression analysis showed that age, prostate volume, PSA velocity, PSA doubling time, and PSA fluctuation rate were risk factors for positive prostate biopsy in patients with two or more PSA tests and each PSA test 4-10 ng/mL (P < 0.05). Based on multivariate logistic regression analysis, a nomogram prediction model was constructed. The area under the ROC curve was 0.859, and the internal validation calibration was good. The slope of the nomogram calibration curve was close to 1, and the Hosmer-Lemeshow goodness of fit test = 20.869, P=0.171.Conclusion The nomogram model constructed based on this study has good discrimination and consistency. It can provide effective help for patients who have two or more PSA tests and each PSA test result 4-10 ng/mL to understand the risk of disease and for urologists to make clinical decisions. -

-
表 1 480例患者临床指标的单因素分析
X±S 临床指标 穿刺结果阳性(213例) 穿刺结果阴性(267例) t P值 年龄/岁 72.53±7.67 66.95±8.41 7.584 < 0.001 BMI/(kg/m2) 23.36±3.21 23.62±3.40 -0.870 0.385 前列腺体积/mL 27.88±16.08 41.29±22.33 -7.375 < 0.001 PSA速度/(ng/mL/年) 2.57±4.22 0.75±0.41 6.980 < 0.001 PSA倍增时间/月 12.68±19.63 32.99±31.01 -8.320 < 0.001 PSA波动率/(%/月) 22.93±12.40 35.27±14.13 -10.033 < 0.001 表 2 各临床指标的多因素logistic结果
因素 β OR 95%CI P值 年龄 -0.104 0.901 0.873~0.928 < 0.001 前列腺体积 0.053 0.949 0.916~0.980 0.002 PSA速度 -0.478 0.620 0.426~0.914 0.016 PSA倍增时间 0.026 1.026 1.012~1.041 < 0.001 PSA波动率 0.123 1.131 1.078~1.195 < 0.001 表 3 列线图模型及各临床指标对PSA波动患者前列腺穿刺阳性的预测效果
临床指标 灵敏度 特异度 约登指数 AUC Z P值 年龄 0.831 0.431 0.262 0.681 -6.806 < 0.001 前列腺体积 0.812 0.554 0.367 0.711 -7.954 < 0.001 PSA速度 0.657 0.723 0.380 0.732 -8.723 < 0.001 PSA倍增时间 0.690 0.783 0.473 0.789 -10.892 < 0.001 PSA波动率 0.770 0.655 0.425 0.745 -9.215 < 0.001 列线图模型 0.803 0.801 0.604 0.859 -
[1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016[J]. CA Cancer J Clin, 2016, 66(1): 7-30. doi: 10.3322/caac.21332
[2] Xia CF, Dong XS, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants[J]. Chin Med J, 2022, 135(5): 584-590. doi: 10.1097/CM9.0000000000002108
[3] Tzeng M, Basourakos SP, Patel HD, et al. Pooled outcomes of performing freehand transperineal prostate biopsy with the PrecisionPoint Transperineal Access System[J]. BJUI Compass, 2022, 3(6): 434-442. doi: 10.1002/bco2.178
[4] 冯天瑞, 严维刚. 经会阴前列腺穿刺活检研究进展[J]. 临床泌尿外科杂志, 2021, 36(6): 485-491. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2021.06.013
[5] 杭天昆, 李通义, 石明凯, 等. 建立针对PSA灰区前列腺癌的临床预测模型: 一项针对SEER数据库的研究[J]. 临床泌尿外科杂志, 2022, 37(8): 606-614. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2022.08.008
[6] Jiang SQ, Huang ZC, Liu BQ, et al. MRI-based nomogram of prostate maximum sectional area and its zone area for prediction of prostate cancer[J]. Front Oncol, 2021, 11: 708730. doi: 10.3389/fonc.2021.708730
[7] Feng F, Zhong YX, Chen Y, et al. Establishment and validation of serum lipid-based nomogram for predicting the risk of prostate cancer[J]. BMC Urol, 2023, 23(1): 120. doi: 10.1186/s12894-023-01291-w
[8] 施云峰, 曹锴, 刘晓武, 等. 认知融合MRI和超声引导靶向穿刺在前列腺前部肿瘤诊断中的临床应用[J]. 临床泌尿外科杂志, 2023, 38(4): 251-254. https://lcmw.whuhzzs.com/article/doi/10.13201/j.issn.1001-1420.2023.04.003
[9] 付振宇, 孙利国, 张鸽, 等. MRI/TRUS认知融合联合12针系统经会阴前列腺穿刺活检的临床研究[J]. 中华男科学杂志, 2021, 27(5): 421-425.
[10] Park YH, Lee JK, Jung JW, et al. Prostate cancer detection rate in patients with fluctuating prostate-specific antigen levels on the repeat prostate biopsy[J]. Prostate Int, 2014, 2(1): 26-30. doi: 10.12954/PI.13037
[11] Celhay O, de la Taille A, Salomon L, et al. Fluctuating prostate-specific antigen levels in patients with initial negative biopsy: should we be reassured?[J]. BJU Int, 2007, 99(5): 1028-1030. doi: 10.1111/j.1464-410X.2007.06752.x
[12] Cormio L, Cindolo L, Troiano F, et al. Development and internal validation of novel nomograms based on benign prostatic obstruction-related parameters to predict the risk of prostate cancer at first prostate biopsy[J]. Front Oncol, 2018, 8: 438. doi: 10.3389/fonc.2018.00438
[13] Kim JS, Ryu JG, Kim JW, et al. Prostate-Specific Antigen fluctuation: what does it mean in diagnosis of prostate cancer?[J]. Int Braz J Urol, 2015, 41(2): 258-264. doi: 10.1590/S1677-5538.IBJU.2015.02.11
[14] Ramírez ML, Nelson EC, Devere White RW, et al. Current applications for prostate-specific antigen doubling time[J]. Eur Urol, 2008, 54(2): 291-300. doi: 10.1016/j.eururo.2008.04.003
[15] Lee SM, Liyanage SH, Wulaningsih W, et al. Toward an MRI-based nomogram for the prediction of transperineal prostate biopsy outcome: a physician and patient decision tool[J]. Urol Oncol, 2017, 35(11): 664. e11-664. e18. doi: 10.1016/j.urolonc.2017.07.018
[16] Hamidi N, Atmaca AF, Canda AE, et al. Does extent of prostate-specific antigen fluctuation can predict Gleason score upgrading in low-risk prostate cancer patients?[J]. Turk J Urol, 2019, 45(Supp. 1): S42-S48.
[17] Flores-Fraile MC, Padilla-Fernández BY, Valverde-Martínez S, et al. The association between prostate-specific antigen velocity(PSAV), value and acceleration, and of the free PSA/total PSA index or ratio, with prostate conditions[J]. J Clin Med, 2020, 9(11): 3400. doi: 10.3390/jcm9113400
[18] Kobayashi M, Kijima T, Yashi M, et al. Prostate-specific antigen kinetics contributes to decision making for biopsy referral: the predictive implication for PSA retest in patients with elevated PSA levels[J]. Prostate Int, 2023, 11(1): 27-33. doi: 10.1016/j.prnil.2022.08.001
[19] Gallagher KM, Christopher E, Cameron AJ, et al. Four-year outcomes from a multiparametric magnetic resonance imaging(MRI)-based active surveillance programme: PSA dynamics and serial MRI scans allow omission of protocol biopsies[J]. BJU Int, 2019, 123(3): 429-438. doi: 10.1111/bju.14513
-




 下载:
下载: