Experience and prognosis analysis of robot-assisted laparoscopic nephron sparing surgery for complex cystic solid renal tumors
-
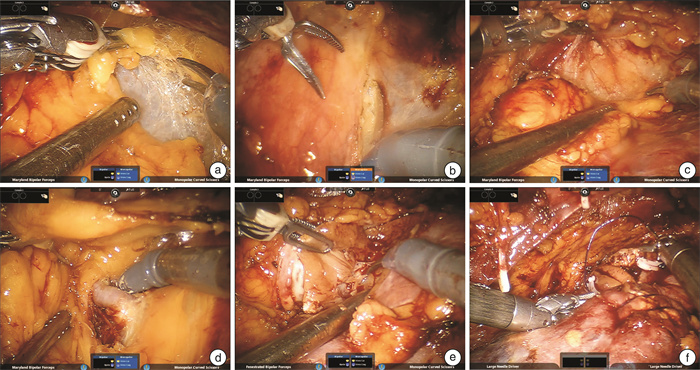
摘要: 目的 探讨机器人辅助腹腔镜保留肾单位手术切除复杂囊实性肾肿瘤的手术经验、安全性并分析患者预后。方法 回顾性分析2013年1月—2023年12月解放军总医院收治的经机器人辅助腹腔镜保留肾单位手术治疗的93例复杂囊实性肾肿瘤患者的临床资料,统计手术成功率、手术时间、肾动脉阻断时间、术中出血量、术中及术后输血量、术后3 d引流量、术后拔管时间、术后住院时间、术前及术后肾小球滤过率(GFR)、围手术期并发症等情况。结果 93例患者中,男68例,女25例;年龄16~74岁,平均(47.66±12.63)岁;肿瘤最大直径0.7~9.0 cm,平均(3.94±1.65)cm;左侧46例,右侧47例。手术全部成功,无中转开放手术。手术时间60~275 min,平均(130.53±46.28) min;肾动脉阻断时间7~68 min,平均(20.84±9.28) min;术中出血量5~550 mL,平均(77.47±110.78) mL,术中输血0例。术后前3 d引流量0~970 mL,平均(64.00±97.79) mL;术后拔管时间2~8 d,平均(3.00±1.07) d;术后住院时间2~14 d,平均(4.62±1.65) d;术前GFR平均为(94.58±16.28) mL/min,术后GFR平均为(83.26±18.45) mL/min;术后患者出现围手术期并发症2例。93例患者的随访时间为3~123个月,中位随访39个月,失访率为11.8%(11/93),复发1例,无肿瘤远处转移。结论 机器人辅助腹腔镜保留肾单位手术治疗复杂囊实性肾肿瘤安全可行,能够有效避免术后并发症,远期肿瘤学控制和恢复良好。
-
关键词:
- 囊实性肾肿瘤 /
- 机器人辅助腹腔镜手术 /
- 保留肾单位手术
Abstract: Objective To evaluate the surgical experience, safety and prognosis of robot-assisted laparoscopic nephron sparing surgery for complex cystic solid renal tumors.Methods The clinical data of 93 patients with complex cystic solid renal tumors who underwent robot-assisted laparoscopic nephron preservation surgery in General Hospital of People's Liberation Army from January 2013 to December 2023 were retrospectively analyzed. The operation success rate, operation time, renal artery blocking time, intraoperative blood loss, intraoperative and postoperative blood transfusion volume, postoperative 3 days' drainage volume, postoperative extubation time, preoperative and postoperative GFR, postoperative hospital stay and perioperative complications were counted.Results Among the 93 patients, 68 were males and 25 were females. The average age was (47.66±12.63) years, ranging from 16 to 74 years. The maximum tumor diameter was 0.7-9.0 cm, with an average of (3.94±1.65) cm, 46 cases on the left side and 47 cases on the right side. All operations were successful without conversion to open surgery. The operation time was 60-275 min, and the average time was (130.53±46.28) min. The time of renal artery occlusion was 7-68 min, and the average time was (20.84±9.28) min. Intraoperative blood loss was 5-550 mL, with an average of (77.47±110.78) mL. There was no case of intraoperative blood transfusion. The postoperative 3 d drainage volume was 0-970 mL, with an average of (64.00±97.79) mL. Postoperative extubation time was 2-8 d, with an average of (3.00±1.07) d. Postoperative hospital stay was 2-14 d, with an average of (4.62±1.65) d. The preoperative GFR was (94.58±16.28) mL/min and the postoperative GFR was (83.26±18.45) mL/min. Perioperative complications occurred in 2 patients. The 93 patients were followed up for a duration ranging from 3 to 123 months, with a median follow-up period of 39 months. The rate of loss to follow-up was recorded at 11.8%(11 out of 93), and only one case of recurrence was observed. No instances of distant metastasis were detected.Conclusion Robot-assisted laparoscopic nephron sparing surgery for complex cystic solid renal tumors is safe and feasible, can effectively avoid postoperative complications, and has good long-term tumor control and recovery. -

-
[1] Jemal A, Bray F, Center MM, et al. Global cancer statistics[J]. CA A Cancer J Clin, 2011, 61(2): 69-90. doi: 10.3322/caac.20107
[2] Campbell SC, Novick AC, Belldegrun A, et al. Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass[J]. J Urol, 2009, 182(4): 1271-1279. doi: 10.1016/j.juro.2009.07.004
[3] Loo RK, Lieberman SF, Slezak JM, et al. Stratifying risk of urinary tract malignant tumors in patients with asymptomatic microscopic hematuria[J]. Mayo Clin Proc, 2013, 88(2): 129-138. doi: 10.1016/j.mayocp.2012.10.004
[4] Bhatt JR, Jewett MAS, Richard PO, et al. Multilocular cystic renal cell carcinoma: pathological T staging makes No difference to favorable outcomes and should be reclassified[J]. J Urol, 2016, 196(5): 1350-1355. doi: 10.1016/j.juro.2016.05.118
[5] Kashan M, Ghanaat M, Hötker AM, et al. Cystic renal cell carcinoma: a report on outcomes of surgery and active surveillance in patients retrospectively identified on pretreatment imaging[J]. J Urol, 2018, 200(2): 275-282. doi: 10.1016/j.juro.2018.02.3087
[6] Hartman DS, Davis CJ Jr, Johns T, et al. Cystic renal cell carcinoma[J]. Urology, 1986, 28(2): 145-153. doi: 10.1016/0090-4295(86)90109-3
[7] Bosniak MA. The Bosniak renal cyst classification: 25 years later[J]. Radiology, 2012, 262(3): 781-785. doi: 10.1148/radiol.11111595
[8] Herts BR, Silverman SG, Hindman NM, et al. Management of the incidental renal mass on CT: a white paper of the ACR incidental findings committee[J]. J Am Coll Radiol, 2018, 15(2): 264-273. doi: 10.1016/j.jacr.2017.04.028
[9] Silverman S, Pedrosa I, Ellis J, et al. Bosniak classification of cystic renal masses, version 2019: an update proposal and needs assessment[J]. Radiology, 2019, 292(2): 475-488. doi: 10.1148/radiol.2019182646
[10] Tse JR, Shen LY, Shen J, et al. Prevalence of malignancy and histopathological association of bosniak classification, version 2019 class Ⅲ and Ⅳ cystic renal masses[J]. J Urol, 2021, 205(4): 1031-1038. doi: 10.1097/JU.0000000000001438
[11] Papadimitriou VG, Takos D, Adamopoulos V, et al. Unusual case of multilocular cystic renal cell carcinoma treated with nephron-sparing technique[J]. G Chir, 2009, 30(8-9): 345-348.
[12] Spaliviero M, Herts BR, Magi-Galluzzi C, et al. Laparoscopic partial nephrectomy for cystic masses[J]. J Urol, 2005, 174(2): 614-619. doi: 10.1097/01.ju.0000165162.21997.11
[13] Pierorazio PM, Patel HD, Feng T, et al. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve[J]. Urology, 2011, 78(4): 813-819. doi: 10.1016/j.urology.2011.04.065
[14] Zhang XH, Shen ZJ, Zhong S, et al. Comparison of peri-operative outcomes of robot-assisted vs laparoscopic partial nephrectomy: a meta-analysis[J]. BJU Int, 2013, 112(8): 1133-1142. doi: 10.1111/bju.12255
[15] Moch H. Cystic renal tumors: new entities and novel concepts[J]. Adv Anat Pathol, 2010, 17(3): 209-214. doi: 10.1097/PAP.0b013e3181d98c9d
[16] Onishi T, Oishi Y, Goto H, et al. Cyst-associated renal cell carcinoma: clinicopathologic characteristics and evaluation of prognosis in 27 cases[J]. Int J Urol, 2001, 8(6): 268-274. doi: 10.1046/j.1442-2042.2001.00298.x
[17] Van Poppel H, Becker F, Cadeddu JA, et al. Treatment of localised renal cell carcinoma[J]. Eur Urol, 2011, 60(4): 662-672. doi: 10.1016/j.eururo.2011.06.040
[18] Volpe A, Blute ML, Ficarra V, et al. Renal ischemia and function after partial nephrectomy: a collaborative review of the literature[J]. Eur Urol, 2015, 68(1): 61-74. doi: 10.1016/j.eururo.2015.01.025
[19] Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904[J]. Eur Urol, 2014, 65(2): 372-377. doi: 10.1016/j.eururo.2013.06.044
[20] Corica FA, Iczkowski KA, Cheng L, et al. Cystic renal cell carcinoma is cured by resection: a study of 24 cases with long-term followup[J]. J Urol, 1999, 161(2): 408-411. doi: 10.1016/S0022-5347(01)61903-7
[21] Fan XX, Xu KW, Lin TX, et al. Comparison of transperitoneal and retroperitoneal laparoscopic nephrectomy for renal cell carcinoma: a systematic review and meta-analysis[J]. BJU Int, 2013, 111(4): 611-621. doi: 10.1111/j.1464-410X.2012.11598.x
[22] Patton MW, Salevitz DA, Tyson MD 2nd, et al. Robot-assisted partial nephrectomy for complex renal masses[J]. J Robot Surg, 2016, 10(1): 27-31. doi: 10.1007/s11701-015-0554-8
[23] Pradere B, Peyronnet B, Delporte G, et al. Intraoperative cyst rupture during partial nephrectomy for cystic renal masses-does it increase the risk of recurrence?[J]. J Urol, 2018, 200(6): 1200-1206. doi: 10.1016/j.juro.2018.06.025
[24] Chen SZ, Wu YP, Chen SH, et al. Risk factors for intraoperative cyst rupture in partial nephrectomy for cystic renal masses[J]. Asian J Surg, 2021, 44(1): 80-86. doi: 10.1016/j.asjsur.2020.03.006
-





 下载:
下载: